Iridopelma marcoi
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Bertani2012ZooKeys230, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Bertani2012ZooKeys230">{{Citation See also the citation download page at the journal. |
Ordo: Araneae
Familia: Theraphosidae
Genus: Iridopelma
Name
Iridopelma marcoi Bertani, 2012 sp. n. – Wikispecies link – ZooBank link – Pensoft Profile
Diagnosis
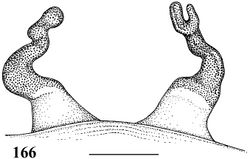
The female resembles that of Iridopelma vanini sp. n. by long double folded spermathecae (Fig. 166), but differ by lacking type II urticating setae on abdomen dorsum. Male unknown.
Etymology
The specific name is a patronym in honour of Marco Antonio de Freitas, a Brazilian zoologist and geographer who collected the holotype and some other aviculariine specimens studied in this work.
Type
Holotype female, Brazil, state of Bahia, São Desidério (12°28'52"S, 45°09'10"W), 724 m a.s.l, M. A. Freitas, October 2009, under tree bark, 1 m above the ground, area of carrasco vegetation (MZSP 36891).
Additional material examined
Only holotype.
Description
Holotype female (MZSP 36891). Carapace 14.9 long, 14.6 wide, chelicerae 9.3. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 12.4, 7.8, 8.9, 8.3, 4.5, 41.9. II: 11.4, 6.9, 8.1, 7.6, 4.4, 38.4. III: 10.0, 6.1, 7.3, 6.5, 4.3, 34.2. IV: 12.2, 6.5, 9.7, 10.1, 4.7, 43.2. Palp: 8.7, 5.5, 5.6, –, 5.9, 25.7. Mid-widths (lateral): femora I–IV = 3.0, 3.1, 3.2, 2.5, palp = 2.4; patellae I–IV = 2.8, 2.8, 2.8, 2.8, palp = 2.4; tibiae I–IV = 2.6, 2.6, 2.5, 2.4, palp = 2.3; metatarsi I–IV = 2.1, 2.0, 2.0, 1.6; tarsi I–IV = 2.2, 2.2, 229, 2.2, palp = 2.2. Abdomen 16.7 long, 11.7 wide. Spinnerets: PMS, 1.9 long, 0.9 wide, 0.3 apart; PLS, 2.3 basal, 1.5 middle, 2.2 distal; mid-widths (lateral), 1.3, 1.2, 1.0, respectively. Carapace: length to width 1.02. Fovea: deep, 2.5 wide. Eyes: tubercle 0.8 high, 2.1 long, 2.6 wide. Clypeus 1.0. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.5, ALE 0.5, PME 0.4, PLE 0.4, AME–AME 0.6, AME–ALE 0.3, AME–PME 0.1, ALE–ALE 1.8, ALE–PME 0.5, PME–PME 1.6, PME–PLE 0.1, PLE–PLE 2.1, ALE–PLE 0.4, AME–PLE 0.4. Ratio of eye group width to length 2.0. Maxillae: length to width: 2.0. Cuspules: 100–150 spread over ventral inner heel. Labium: 1.9 long, 2.6 wide, with ca. 150 cuspules. Labio-sternal groove deep, narrow, sigilla not evident. Chelicerae: basal segments with ten teeth decreasing in size from distal to basal portion. Sternum: 7.5 long, 6.6 wide. Legs: leg formula: I=IV II III. Scopula: tarsi I–IV fully scopulate. Metatarsi I–II 4/5 scopulate; III 2/3, IV 1/2 distal scopulate. IV divided by three wide row of setae. Urticating hairs lacking on abdomen dorsum. Genitalia: paired long and converging spermathecae tapering strongly from base to apex, double folded and with a strong distal constriction forming one or two distal lobes (Fig. 166). Color pattern: carapace, chelicerae, and femora of legs and palps dark brown covered with dense layer of metallic green setae. Other leg and palp segments dorsally dark brown with brown setae. Tibiae and patellae covered with metallic blue setae prolaterally and retrolaterally; ventrally black. Tarsi with a dorsal “U” orange stripe. Sternum, labium, maxillae and coxae black. Abdomen ventrally black; dorsally dark brown with a large lighter central spot. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi inconspicuous. Distal femora, patellae, tibiae and metatarsi with broad white rings (Fig. 167).
Male: unknown.
Distribution
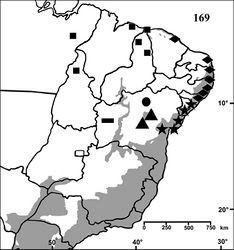
Brazil: known only from São Desidério, state of Bahia (Fig. 169).
Natural history
The only known specimen was found in cerrado/carrasco vegetation (Fig. 168), under loose tree bark about one meter above the ground (M. A. Freitas, pers. comm.).
Color pattern ontogeny
Only the adult female holotype is known.
Cladistics
Searches using NONA resulted in 12 trees and strict consensus is used (Fig. 177). With X-PEE-WEE, it was found from 1 to 6 trees, depending on the concavity used (Table 2). Cladogram obtained with concavity 6 is shown in Figs 178. The main difference of cladograms obtained with the different strategies comprise the position of clade having Ephebopus, Tapinauchenius and Psalmopoeus. In NONA and X-PEE-WEE with concavities 2–6 that clade is paraphyletic in respect to other aviculariine genera (Figs 177–178); with X-PEE-WEE and concavity 1 Aviculariinae is retrieved as monophyletic. Topologies of clade Typhochlaena (Avicularia spp.1 (Pachistopelma, Avicularia spp.2, Iridopelma)) were retrieved in all employed search strategies. Resolution of the terminal clade retrieving Iridopelma as sister group of Avicularia spp.2 was obtained with X-PEE-WEE and concavities 1–5, whereas Iridopelma and Avicularia spp.2 were in a tricotomy with Pachistopelma when using NONA and X-PEE-WEE with concavity 6. Internal relationship of Typhochlaena is totally unresolved with NONA, and partially resolved with X-PEE-WEE, independently of the concavity used. Position of Iridopelma marcoi sp. n. and Iridopelma katiae sp. n. change more markedly. With concavities 1–5 Iridopelma katiae sp. n. is sister-group of Pachistopelma (Iridopelma + Avicularia spp.2). With NONA and X-PEE-WEE with concavity 6 it is part of Iridopelma clade. Iridopelma marcoi sp. n. is part of Iridopelma clade only with NONA. With X-PEE-WEE it is always polyphyletic. Its position will be fully discussed below.
| Trees | Length | FIT | |
|---|---|---|---|
| K = 1 | 2 | 221 | 2945,4 |
| K = 2 | 6 | 213 | 3549,1 |
| K = 3 | 6 | 213 | 3919,8 |
| K = 4 | 6 | 213 | 4172,5 |
| K = 5 | 1 | 211 | 4361,2 |
| K = 6 | 1 | 209 | 4507,4 |
In agreement with other studies (Ramírez 2003[2], West et al. 2008[1], Bertani et al. 2011[3]) the shortest tree was obtained with X-PEE-WEE and concavity 6 (Fig. 178, Tables 3–4). It has also the highest fit (Table 2) and it is chosen as the preferred tree, on which the discussion below is done.
| Character | Fit | Steps | Extra Steps | Character | Fit | Steps | Extra Steps |
|---|---|---|---|---|---|---|---|
| 0 | 50 | 7 | 6 | 31 | 100 | 2 | 0 |
| 1 | 66.7 | 4 | 3 | 32 | 42.86 | 9 | 8 |
| 2 | 54.5 | 7 | 5 | 33 | 75 | 3 | 2 |
| 3 | 100 | 2 | 0 | 34 | 75 | 3 | 2 |
| 4 | 85.71 | 2 | 1 | 35 | 100 | 2 | 0 |
| 5 | 60 | 5 | 4 | 36 | 85.71 | 2 | 1 |
| 6 | 85.71 | 3 | 1 | 37 | 100 | 1 | 0 |
| 7 | 100 | 1 | 0 | 38 | 85.71 | 2 | 1 |
| 8 | 85.71 | 2 | 1 | 39 | 60 | 6 | 4 |
| 9 | 100 | 1 | 0 | 40 | 54.54 | 7 | 5 |
| 10 | 100 | 1 | 0 | 41 | 54.54 | 7 | 5 |
| 11 | 85.71 | 2 | 1 | 42 | 85.71 | 2 | 1 |
| 12 | 50 | 8 | 6 | 43 | 85.71 | 3 | 1 |
| 13 | 40 | 11 | 9 | 44 | 85.71 | 2 | 1 |
| 14 | 75 | 5 | 2 | 45 | 85.71 | 2 | 1 |
| 15 | - | - | - | 46 | 85.71 | 2 | 1 |
| 16 | 66.67 | 5 | 3 | 47 | 85.71 | 2 | 1 |
| 17 | 85.71 | 2 | 1 | 48 | 66.67 | 6 | 3 |
| 18 | - | - | - | 49 | 100 | 1 | 0 |
| 19 | 85.71 | 2 | 1 | 50 | 75 | 3 | 2 |
| 20 | 100 | 1 | 0 | 51 | 85.71 | 3 | 1 |
| 21 | 75 | 3 | 2 | 52 | - | - | - |
| 22 | 85.71 | 2 | 1 | 53 | 100 | 1 | 0 |
| 23 | - | - | - | 54 | 100 | 1 | 0 |
| 24 | 60 | 6 | 4 | 55 | - | - | - |
| 25 | 66.67 | 4 | 3 | 56 | 100 | 1 | 0 |
| 26 | 100 | 1 | 0 | 57 | 85.71 | 3 | 1 |
| 27 | 50 | 7 | 6 | 58 | 75 | 4 | 2 |
| 28 | 85.71 | 2 | 1 | 59 | 50 | 9 | 6 |
| 29 | 75 | 8 | 2 | 60 | 75 | 5 | 2 |
| 30 | 85.71 | 2 | 1 | 61 | 85.71 | 4 | 1 |
| Taxa or Node | Character | Change | Taxa or Node | Character | Change | Taxa or Node | Character | Change |
|---|---|---|---|---|---|---|---|---|
| Holothele | 3 | 0 → 1 | Ephebopus cyanognathus | 13 | 1 → 2 | 29 | 0 → 4 | |
| 32 | 0 → 1 | 25 | 0 → 1 | 50 | 0 → 1 | |||
| 39 | 0 → 1 | Ephebopus foliatus | 27 | 0 → 1 | 60 | 3 → 2 | ||
| Heteroscodra | 12 | 1 →0 | Ephebopus rufescens | 60 | 3 → 2 | Node 46 | 24 | 1 → 2 |
| Pterinochilus | 2 | 1 → 2 | Avicularia sp.1 | 24 | 1 → 2 | Node 47 | 51 | 0 → 2 |
| 13 | 1 → 2 | Avicularia diversipes | 24 | 1 → 2 | 56 | 0 → 1 | ||
| 19 | 0 → 1 | 33 | 0 → 1 | Node 48 | 14 | 2 → 3 | ||
| 58 | 0 → 1 | 40 | 1 → 2 | 28 | 0 → 1 | |||
| 59 | 0 → 2 | Avicularia sooretama | 32 | 0 → 1 | Node 49 | 2 | 1 → 0 | |
| Pelinobius | 2 | 0 → 2 | 39 | 0 → 1 | 16 | 0 → 1 | ||
| 12 | 1 → 0 | Avicularia taunayi | 32 | 0 → 1 | 39 | 0 → 1 | ||
| 13 | 1 → 0 | 59 | 0 → 1 | Node 50 | 12 | 1 → 0 | ||
| 15 | 0 → 1 | Typhochlaena seladonia | 0 | 1 → 0 | 13 | 1 → 0 | ||
| 17 | 0 → 1 | 16 | 2 → 1 | 27 | 1 → 0 → 1 | |||
| 22 | 0 → 1 | 24 | 1 → 0 | Node 51 | 5 | 0 → 1 | ||
| 32 | 0 → 1 | 25 | 0 → 1 | 33 | 0 → 1 | |||
| 58 | 0 → 1 | Typhochlaena amma | 12 | 1 → 0 | 39 | 0 → 2 | ||
| 59 | 0 → 2 | 25 | 0 → 1 | Node 52 | 36 | 1 → 0 | ||
| Haplopelma | 2 | 1 → 2 | 33 | 0 → 1 | Node 53 | 29 | 4 → 6 | |
| 8 | 0 → 1 | Typhochlaena curumim | 12 | 1 → 0 | Node 54 | 0 | 1 → 0 | |
| 19 | 0 → 1 | 32 | 0 → 1 | 1 | 0 → 1 → 0 | |||
| 38 | 0 → 1 | Typhochlaena paschoali | 16 | 2 → 1 | 2 | 1 → 0 | ||
| 47 | 0 → 1 | 32 | 0 → 1 | 9 | 0 → 1 | |||
| 48 | 3 → 2 | Typhochlaena costae | 59 | 0 → 1 | 12 | 1 → 0 | ||
| 60 | 1 → 3 | Iridopelma hirsutum | 35 | 1 → 0 | 13 | 1 → 0 | ||
| Phlogiellus | 0 | 1 → 0 | Iridopelma vanini | 5 | 0 → 1 | 24 | 1 → 0 | |
| 6 | 0 → 2 | Iridopelma katiae | 30 | 1 → 0 | 40 | 1 → 0 | ||
| 11 | 1 → 0 | 59 | 0 → 3 | 61 | 1 → 3 | |||
| 21 | 0 → 1 | 61 | 1 → 3 | Node 55 | 34 | 0 → 1 | ||
| Lasiodora | 2 | 1 → 2 | Iridopelma marcoi | 25 | 0 → 1 | Node 56 | 29 | 4 → 5 |
| 18 | 0 → 1 | 27 | 1 → 0 | Node 58 | 7 | 0 → 1 | ||
| 22 | 0 → 1 | 32 | 0 → 1 | 10 | 0 → 1 | |||
| 31 | 0 → 1 | 34 | 0 → 1 | Node 59 | 59 | 0 → 1 | ||
| 41 | 0 → 1 | 59 | 0 → 1 | Node 60 | 34 | 0 → 1 | ||
| 42 | 0 → 1 | Iridopelma oliveirai | 27 | 1 → 0 | Node 61 | 32 | 0 → 1 | |
| 43 | 0 → 1 | Encyocratella | 4 | 0 → 1 | Node 62 | 29 | 4 → 5 | |
| 46 | 0 → 1 | 5 | 0 → 1 | 41 | 2 → 0 | |||
| 47 | 0 → 1 | 6 | 0 → 1 | 49 | 0 → 1 | |||
| 52 | 0 → 1 | 17 | 0 → 1 | Node 64 | 30 | 0 → 1 | ||
| Euathlus | 6 | 0 → 1 | 58 | 0 → 1 | 61 | 0 → 1 | ||
| 13 | 1 → 2 | Node 38 | 0 | 1 → 0 | Node 65 | 28 | 0 → 1 | |
| 29 | 0 → 3 | 1 | 0 → 1 | 36 | 0 → 1 | |||
| 32 | 0 → 1 | 4 | 0 → 1 | Node 66 | 29 | 1 → 4 | ||
| 55 | 0 → 1 | 26 | 0 → 1 | 40 | 0 → 1 | |||
| 59 | 0 → 1 | 50 | 0 → 1 | 51 | 0 → 1 | |||
| Poecilotheria | 0 | 1 → 0 | 58 | 0 → 2 | 53 | 0 → 1 | ||
| 14 | 2 → 3 | Node 39 | 3 | 0 → 2 | Node 68 | 14 | 2 → 3 | |
| 16 | 0 → 2 | 8 | 0 → 1 | 16 | 0 → 2 | |||
| 21 | 0 → 1 | Node 40 | 20 | 0 → 1 | Node 69 | 60 | 3 → 1 | |
| 29 | 1 → 2 | 40 | 0 → 1 | Node 70 | 27 | 0 → 1 | ||
| 41 | 0 → 1 | 46 | 0 → 1 | 29 | 0 → 1 | |||
| 59 | 0 → 1 | Node 41 | 42 | 0 → 1 | Node 71 | 37 | 0 → 1 | |
| Psalmopoeus | 13 | 1 → 2 | 44 | 0 → 1 | Node 72 | 0 | 0 → 1 | |
| 21 | 0 → 1 | 45 | 0 → 1 | 11 | 0 → 1 | |||
| 40 | 0 → 1 | Node 42 | 38 | 0 → 1 | 12 | 0 → 1 | ||
| Tapinauchenius | 39 | 1 → 0 | 44 | 0 → 1 | 13 | 0 → 1 | ||
| Ephebopus murinus | 12 | 1 → 2 | 45 | 0 → 1 | 24 | 0 → 1 | ||
| 13 | 1 → 2 | 51 | 0 → 1 | Node 73 | 2 | 0 → 1 | ||
| 23 | 0 → 1 | 54 | 0 → 1 | 60 | 0 → 3 | |||
| 57 | 1 → 2 | Node 43 | 0 | 1 → 0 |
Pachistopelma is monophyletic and supported by 9 synapomorphies: low eye tubercle (character 0), straight anterior row of eyes (character 1), clypeus absent (character 2), flattened abdomen a quarter longer than wide in females (character 9), leg IV longer than leg I in females (character 12) and in males (character 13), leg rings on distal tibiae and metatarsi not evident (character 24), embolous straight or very slightly curved (character 40), bromelicolous lifestyle (61). All these synapomorphies are very homoplasious (3 additional steps or more), except for character 9, which is exclusive for the genus, and character 61, homoplastic with Iridopelma katiae sp. n.
Iridopelma is monophyletic only if Iridopelma marcoi sp. n. is excluded. However, some important characters for the analyses are exclusive to males (i. e., tibial spurs, cymbium protuberance) and at least one, exclusive to Iridopelma (tibial spur presence on leg II). The lack of a known male in this species undoubtedly influenced its position in the cladogram topology. Also, the absence of urticating hairs in female, that is an important synapomorphy for a large aviculariine clade, surely contributed for this result as well. On the other hand, the spermathecae shape, as well as some somatic characters indicate it is an Iridopelma species. The discovery of the male of Iridopelma marcoi sp. n. migth confirm its position.
Other Iridopelma species are in a monophyletic clade. Three synapomorphies support Iridopelma monophyly: dorsal abdominal pattern in immatures (Character 29), homoplasious with Typhochlaena amma sp. n.; embolous length that changes from long to median (Character 41), extremally homoplasious, and presence of a tibial spur also on leg II, exclusive for the genus (character 49). Therefore, the main synapomorphy for Iridopelma is still the classical character proposed by Pocock (1901)[4]. However, the other proposed synapomorphy, leg I longer than leg IV (Pocock 1901[4]) (character 12 for females and 13 for males) is extremely homoplasious. Even in Iridopelma, only Iridopelma hirsutum and Iridopelma oliveirai sp. n. males have a clearly longer leg I in relation to leg IV. All Iridopelma females have leg I and IV of similar length. Support for the node 59 (Iridopelma vanini sp. n.+ Iridopelma oliveirai sp. n.) is their habitat in deciduous forest (character 59), an homoplasious character. Node 60 Iridopelma hirsurtum (Iridopelma vanini sp. n. + Iridopelma oliveirai sp. n.) has as apomorphy the spiraled spermathecae (character 34). Node 61 is supported by character 32, presence of lobes in spermathecae, extremely homoplasious.
Biogeography
Typhochlaena, Pachistopelma and Iridopelma species are highly endemic, and there is no overlapping in distribution of species belonging to the same genus (Figs 179–181). Most areas of endemism for species of those genera are mostly concordant with river systems, as proposed for Atlantic rainforest in Northeastern and Southeastern Brazil (Pellegrino et al. 2005[5]). The northermost endemic area ranges roughly from state of Ceará/Rio Grande do Norte southwards to state of Alagoas and is limited in the south by Rio São Francisco. Iridopelma hirsutum, Pachistopelma rufonigrum and Typhochlaena curumim sp. n. distribution is restricted to this region (Figs 179–181). Southwards, another area of endemism is recognized between Rio São Francisco and Rio Paraguaçú (Pellegrino et al. 2005[5]). Iridopelma zorodes, Pachistopelma bromelicola sp. n. and Typhochlaena seladonia are found in this endemic area, but there are some records for Pachistopelma bromelicola sp. n. south of Rio Paraguaçú (Elísio Medrado and Maracás, state of Bahia). Cladistic analysis retrieved Typhochlaena seladonia/Typhochlaena curumim sp. n. and Pachistopelma rufonigrum/Pachistopelma bromelicola sp. n. as sister species, indicating Rio São Francisco and Rio Paraguaçú as potential barriers responsible for vicariant event (Figs 179–180). However, the analysis did not retrieve Iridopelma zorodes as sister species of Iridopelma hirsutum (Fig. 181), suggesting a more complex history for Iridopelma genus in Northeastern and Center-Western Brazil. Another area of endemism was proposed for the region from Rio Paraguaçú southwards to Rio Jequitinhonha (Pellegrino et al. 2005[5]). Typhochlaena paschoali sp. n., Avicularia diversipes and Avicularia gamba are endemic to the region (Figs 179, 182). Following southwards, another area of endemism is proposed between Rio Jequitinhonha and Rio Doce (Pellegrino et al. 2005[5]). Only Avicularia sooretama is known from this region. Interestingly, Rio Doce is not a barrier for the species, which is distributed from Rio Jequitinhonha to Rio Paraíba do Sul (Fig. 182). However, there is a single old record for the species south of Rio Doce, in Itatiaia, State of Rio de Janeiro (Bertani and Fukushima 2009[6]). The southermost area of endemism for aviculariines is between Rio Doce and Rio Paraíba do Sul. Typhochlaena amma sp. n. is endemic to this region and cladistic analysis retrieved it as sister species of Typhochlaena paschoali sp. n. (Fig. 179).
At least two hypotheses were proposed to explain the existence of endemism areas in Atlantic forest of Northeastern and Southeastern Brazil. Pellegrino et al. (2005)[5] proposed that after a semiarid climate in Miocene/Pliocene boundary the region had higher humidity conditions in the Pliocene, and major rivers were formed, bissecting deposits from Barreiras Formation (Sugio and Nogueira 1999), thus isolating inter-riverine regions. The alternative hypothesis (Carnaval and Moritz 2008[7]) provided evidence for the existence of forest refugia areas during the Quaternary that are congruent with areas of endemism known for Atlantic forest of Northeastern Brazil.
Whereas distribution of congeners did not overlap, there is strong ovelap of species of Typhochlaena with Pachistopelma, Iridopelma and Avicularia spp.2 as well as of Iridopelma with Pachistopelma (Fig. 183). Typhochlaena spp. overlap with Avicularia spp. 2 on state of Espírito Santo and Southern state of Bahia, with Iridopelma on Northern state of Bahia, Sergipe, Paraíba, Piauí, Maranhão and possibly Pernambuco, Alagoas and Tocantins. They also overlap with Pachistopelma spp. in Northern Bahia, Sergipe, Alagoas, Pernambuco and Paraíba. Pachistopelma spp. overlap with Iridopelma spp. and Typhochlaena spp. over almost all their distribution (from Rio Grande do Norte to Northern Bahia). Iridopelma possibly overlaps slightly with Avicularia spp.1 in parts of Maranhão, Pará and Tocantins, with Typhochlaena and Pachistopelma on the coast and with Typhochlaena on Northern Bahia, Sergipe, Alagoas, Pernambuco, Paraiba and Southern Ceara, Piauí, Maranhão and parts of Tocantins. Most of the overlap involves Typhochlaena – the most basal genus of the clade, and, if this genus is not considered, only a marginal overlapping of Avicularia spp.1, Avicularia spp.2 and Iridopelma will remain (except Pachistopelma, which will be discussed separately).
Habitat and evolution
The overlap of Iridopelma spp. with Pachistopelma spp. in most of the distribution of the latter deserves a more detailed discussion. Pachistopelma spp. are strictly associated with bromeliads (Bertani et al. 1994[8], Santos et al. 2002[9], 2004[10]; Dias et al. 2000[11], Dias and Brescovit 2003[12], Dias and Brescovit 2004[13], this work), a characteristic possibly shared only with Iridopelma katiae sp. n. In all other closely related aviculariine genera with available information, the retreat is made under loose tree bark (Typhochlaena seladonia, Typhochlaena curumim sp. n.), on tree trunk or on leaves of palm trees (Avicularia sp.1, Avicularia juruensis and Avicularia taunayi), with two or more leaves connected with silk threads (Avicularia spp. 2, Iridopelma hirsutum, Iridopelma zorodes). Therefore, almost all other aviculariine species use primarily trees to make their retreats, though some use bromeliads eventually (immature Avicularia avicularia - Stradling, 1994; Iridopelma oliveirai sp. n.– type label data; immature Avicularia juruensis, immature and adult Avicularia diversipes (pers. obs.).
Pachistopelma spp. inhabit tank bromeliads - Aechmea aquilega (Dias et al. 2000[11], Santos et al. 2004[10]); Hohenbergia stellata, Hohenbergia ridley (Dias et al. 2000[11]); Hohenbergia ramageana and Avicularia lingulata (Santos et al. 2004[10]) which are mainly terrestrial in rocky outcrops, restinga and even caatinga, and are facultatively epiphytes (Siqueira Filho and Leme 2006[14]). When terrestrials, they grow on areas of shallow, sandy or rocky soil, exposed to intense sunlight. Tank bromeliads retain rain water on their phytothemalta and are an important source of humidity to several species of animals (Frank and Lounibos 2009[15]). Tank bromeliads are particularly abundant in drier areas of restinga which are depleted of high trees (Santos et al. 2004[10]). In this xeric environment there are few available places with adequate humidity and protection against high temperatures. Therefore, bromeliads provide the few suitable microhabitats for a variety of animals.
Pachistopelma spp. seem specialized to live inside bromeliads, mainly by the dorso-ventrally flattened body of immatures and female which aids the spider in moving between the narrow inter-leaves spaces (Bertani 1994, Bertani in Dias et al. 2000[11]). A question arising then is, why this specialization occurred with Pachistopelma spp., but not with Iridopelma spp. that live in contiguous areas? It is largely known that climate fluctuations of the Neogene and the Quaternary periods transformed large wet forested regions of Northeastern Brazil into xeric biomes (Ab’Sáber 1977, Martin et al. 1993[16], Suguio and Nogueira 1999[17], Carnaval and Moritz 2008[7]). With the change, part of the fauna more dependent on wet and low temperature probably became extinct. Especially arboreal animals might be affected, as large trees became rare. On the other hand, bromeliads, that live normally as epiphytes or on rocks and forest borders, expanded their distribution on the ground. Thus, these plants were the few available places for the ancestor of Pachistopelma species to live. Natural selection then acted and morphological modifications took place leading to speciation and specialization to live strictly inside bromeliads. When climate changed and the region became wet again, the forest expanded and bromeliads became restricted as epiphytes or more concentrated in restinga regions in the coast, caatinga, or on rocky formations on hills. Even though the habitat along their distribution area is now suitable for an arboreal spider, the morphological, ethological end physiological specializations of Pachistopelma species did not allow them to recolonize trees. Pachistopelma populations seem to be smaller in bromeliads on shaded forest borders than in regions exposed to direct sunlight (Santos et al. 2004[10], pers. obs.). On the other hand, Iridopelma species expanded their distribution following forest expansion and now species of the two genera have sympatric distribution, one living inside bromeliads and the other in trees. Existence of other animal species living strictly inside bromeliads in the same region reinforces the idea. At least another spider, Nothroctenus fuxico Dias and Brescovit, 2004 is found exclusively in bromeliads, sometimes together with Pachistopelma bromelicola sp. n. specimens (Dias and Brescovit 2004[13]). One scorpion, Tityus neglectus Mello-Leitão, 1932, is also found living inside tank bromeliads (Lourenço and Eickstedt 1988[18], Santos et al. 2003[19]) in same regions as Pachistopelma spp. This model basically corresponds to the ecogeographical speciation proposed by Vanzolini and Williams (1981)[20].
At least five other aviculariine species are found in open vegetation regions - cerrado (Typhochlaena costae sp. n., Iridopelma marcoi sp. n., Iridopelma vanini sp. n.), caatinga (Iridopelma oliveirai sp. n.), sandy dunes/restinga (Iridopelma vanini sp. n.), or campo rupestre (Iridopelma katiae sp. n.). For all species with available field data, there are records of them living on trees (Iridopelma marcoi sp. n.) or in bromeliads (Iridopelma oliveirai sp. n., Iridopelma katiae sp. n.). The holotype of Iridopelma vanini sp. n. was found under a fallen tree trunk on a sandy dune region in Parnaíba, state of Piaui. Iridopelma katiae sp. n. habitat is interesting, because it is in a high region (1200–1300 m a.s.l.) in a campo rupestre area. As restinga and caatinga, the climate is severe, with rocky formations, water stress present most times of year, high temperatures during the day and low temperatures at night, and few sparse trees (Conceição et al. 2007[21]). Again, the only available place for an arboreal spider is the frequent bromeliad islands formed by Vriesea atra. The four specimens collected in this region were inside those bromeliads, including a female with spiderlings. The other two records are for a region close to this and the spiders were found under rocks, an unusual habitat for an arboreal spider. This indicates that Iridopelma katiae sp. n. could be suffering a similar selective pressure that led to the specialization to bromeliad lifestyle in Pachistopelma spp., after a climatic and vegetational change.
Another bromelicolous spider, the salticid Psecas chapoda (Peckham & Peckham, 1894), is specialized to living inside the bromeliad Bromelia balanseae (Romero and Vasconcelos-Neto 2005[22]). These bromeliads also occur in open vegetation formation (cerrado), which is, sometimes, bordered by forest formations. Psecas chapoda is physiologically adapted to Bromelia balanseae, and denser populations’ occur far from shaded areas of forest borders (Romero and Vasconcelos-Neto 2005[22]). A facultative mutualism involving Psecas chapoda and Bromelia balanseae has been proposed, in which the plant benefits from nutrients generated by spider debris, such as feces, exuvia, prey carcasses and silk (Romero et al. 2006[23], Romero et al. 2008[24]). A similar process to that of Pachistopelma spp. could have occurred with an ancestor of this salticid spider, leading to a specialization to live inside bromeliads. Salticid association with bromeliads are much more frequent in bromelids of open environments, such as cerrado, rather than in bromeliads of forest interiors (Romero 2006[25]). As with cerrado, campo rupestre is another key area to understanding the association between spiders and bromeliads. Alpaida quadrilorata (Simon, 1897) (Araneidae) is also associated with a bromeliad-like plant, Paepalanthus bromelioides (Eriocaulaceae) (Figueira and Vasconcellos-Neto 1991[26]) which also has leaves in a rosette and occurs on sandy soil in campo rupestre areas of Serra do Cipó. I also observed the same association with these spider species and plant in campo rupestre of Caraça (Santa Bárbara, state of Minas Gerais). Therefore, this can be a recurrent process leading to the strict bromelicous lifestyle, and should be investigated in other animal groups having similar habits.
This complex history may be responsible for the extraordinary diversity of aviculariines in Northeastern, Southeastern and Central-western Brazil, which rivalizes with the aviculariine forms found in Northern South America and Central America in number of species, morphological variation and habitat use. Specifically, parts of Northeastern Brazil, as Reconcavo Bahiano, has one of the richest aviculariine fauna in the world, with records for Avicularia diversipes, Avicularia gamba, Iridopelma zorodes, Pachistopelma bromelicola sp. n., and possibly at least one Typhochlaena species.
Due to their limited dispersal abilities and high endemism, theraphosids are important taxa for biogeographic and evolutionary studies. Phylogeographical studies with species of aviculariines would aid in understanding important evolutionary processes and biogeographical patterns in South America, and I expect the present paper stimulates this kind of study with these animals.
Conservation
As with other Brazilian Atlantic forest aviculariines (Avicularia diversipes, Avicularia sooretama and Avicularia gamba – Bertani and Fukushima 2009[6]), species of Typhochlaena, Iridopelma and Pachistopelma are very endemic. Typhochlaena species are, moreover, rare and little is known about their habits. Only forty specimens are known in collections for all five species. As Typhochlaena is the sister group of a large aviculariine clade, the conservation of the relict species of this genus is imperative to preserve aviculariine diversity (Vane-Wright et al. 1991, Faith 1992[27]).
Iridopelma hirsutum and Iridopelma zorodes are abundant, but they are known only from patches of Atlantic forest, which is limited to 7.91% of its original distribution (Fundação SOS Mata Atlantica and INPE 2009[28]). These species depend on forest protection as a whole. Little data is available on Iridopelma oliveirai sp. n., Iridopelma vanini sp. n., Iridopelma marcoi sp. n. and Iridopelma katiae sp. n. All these species occur in drier areas in cerrado, caatinga, campo rupestre and restinga and can be under natural stress. Information on population sizes and habitat usage are desirable to aid in conservation of these species. Pachistopelma spp. populations are normally high, but their existence, as a specialized species, is dependent upon conservation of bromeliads, mainly in restinga of Northeastern Brazil. However, bromeliad species are also threatened in several portions of that region (Siqueira Filho and Tabarelli 2006[14]).
Original Description
- Bertani, R; 2012: Revision, cladistic analysis and biogeography of Typhochlaena C. L. Koch, 1850, Pachistopelma Pocock, 1901 and Iridopelma Pocock, 1901 (Araneae, Theraphosidae, Aviculariinae) ZooKeys, 230: 1-94. doi
Other References
- ↑ 1.0 1.1 1.2 1.3 1.4 West R, Marshall S, Fukushima C, Bertani R (2008) Review and cladistic analysis of the Neotropical tarantula genus Ephebopus Simon 1892 (Araneae: Theraphosidae) with notes on the Aviculariinae. Zootaxa 1849: 35–58. http://www.mapress.com/zootaxa/2008/f/z01849p058f.pdf
- ↑ Ramírez M (2003) The spider subfamily Amaurobioidinae (Araneae, Anyphaenidae): a phylogenetic revision at the generic level. Bulletin of the American Museum of Natural History 277: 1–262. http://digitallibrary.amnh.org/dspace/handle/2246/445 doi: <0001:TSSAAA>2.0.CO;2 10.1206/0003-0090(2003)277<0001:TSSAAA>2.0.CO;2
- ↑ Bertani R, Nagahama R, Fukushima C (2011) Revalidation of Pterinopelma Pocock 1901 with description of a new species and the female of Pterinopelma vitiosum (Keyserling 1891) (Araneae: Theraphosidae: Theraphosinae). Zootaxa 2814: 1–18. http://www.mapress.com/zootaxa/2011/f/zt02814p018.pdf
- ↑ 4.0 4.1 Pocock R (1901) Some new and old genera of South American Aviculariidae. Annals and Magazine of Natural History 7: 540-555. doi: 10.1080/03745480109443359
- ↑ 5.0 5.1 5.2 5.3 5.4 Pellegrino K, Rodrigues M, Waite A, Morando M, Yassuda Y, Sites JR J (2005) Phylogeography and species limits in the Gymnodactylus darwinii complex (Gekkonidae, Squamata): genetic structure coincides with river systems in the Brazilian Atlantic Forest. Biological Journal of the Linnean Society 85: 13-26. doi: 10.1111/j.1095-8312.2005.00472.x
- ↑ 6.0 6.1 Bertani R, Fukushima C (2009) Description of two new species of Avicularia Lamarck 1818 and redescription of Avicularia diversipes (C.L. Koch 1842) (Araneae, Theraphosidae, Aviculariinae) – three possibly threatened Brazilian species. Zootaxa 2223: 25–47. http://www.mapress.com/zootaxa/2009/f/zt02223p047.pdf
- ↑ 7.0 7.1 Carnaval A, Moritz C (2008) Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. Journal of Biogeography 35: 1187-1201. doi: 10.1111/j.1365-2699.2007.01870.x
- ↑ Bertani R, Silva Júnior P, Lucas S (1994) Revisão dos gêneros da subfamília Aviculariinae (Araneae, Mygalomorphae, Theraphosidae). Resumos do XX Congresso Brasileiro de Zoologia, Rio de Janeiro: 73–74.
- ↑ Santos R, Almeida M, Nunes J (2002) Notes on the association of Pachistopelma rufonigrum Pocock, 1901 (Theraphosidae) with phytotelm bromeliads in eastern Rio Grande do Norte State, NE-Brazil. Journal of the Bromeliad Society 52: 122-124.
- ↑ 10.0 10.1 10.2 10.3 10.4 Santos R, Almeida M, Tinoco L, Martins L, Maia M (2004) Biogeography and conservation of the bromeliad tarantula Pachistopelma rufonigrum (Araneae, Theraphosidae) in Rio Grande do Norte, Brazil. Journal of the Bromeliad Society 54: 153-157.
- ↑ 11.0 11.1 11.2 11.3 Dias S, Brescovit A, Santos L, Couto E (2000) Aranhas em bromélias de duas restingas do estado de Sergipe, Brasil. Biologia Geral e Experimental 1: 22–24. http://www.biologiageralexperimental.bio.br/temas/aranhas/1.pdf
- ↑ Dias S, Brescovit A (2003) Notes on the behavior of Pachistopelma rufonigrum Pocock (Araneae, Theraphosidae, Aviculariinae). Revista Brasileira de Zoologia 20: 13–17. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-81752003000100004&lng=pt&nrm=iso&tlng=en doi: 10.1590/S0101-81752003000100004
- ↑ 13.0 13.1 Dias S, Brescovit A (2004) Microhabitat selection and co-occurrence of Pachistopelma rufonigrum Pocock (Araneae, Theraphosidae) and Nothroctenus fuxico sp. nov. (Araneae, Ctenidae) in tank bromeliads from Serra de Itabaiana, Sergipe, Brazil. Revista Brasileira de Zoologia 21: 789–796. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-81752004000400011&lng=pt&nrm=iso&tlng=en doi: 10.1590/S0101-81752004000400011
- ↑ 14.0 14.1 Siqueira Filho J, Tabarelli M (2006) Bromeliad species of the Atlantic forest of north-east Brazil: losses of critical populations of endemic species. Oryx 40: 218-224. doi: 10.1017/S0030605306000627
- ↑ Frank J, Lounibos L (2009) Insects and allies associated with bromeliads: a review. Terrestrial Arthropod Reviews 1: 125-153. doi: 10.1163%2f187498308X414742
- ↑ Martin L, Suguio K, Flexor J (1993) As flutuações de nível do mar durante o Quaternário superior e a evolução geológica de deltas brasileiros. Boletim IG-USP, Publicação Especial 15: 1-186.
- ↑ Suguio K, Nogueira A (1999) Revisão crítica dos conhecimentos geológicos sobre a formação (ou grupo?) Barreiras do Neógeno e o seu possível significado como testemunho de alguns eventos geológicos mundiais. Geociências, S. Paulo 18: 461-479.
- ↑ Lourenço W, Eickstedt V (1988) Sinopse das espécies de Tityus do Nordeste do Brasil, com a redescrição da Typhochlaena neglectus Mello-Leitão (Scorpiones, Buthidae). Revista Brasileira de Zoologia 5: 399–408. http://www.scielo.br/scielo.php?script=sci_abstract&pid=S0101-81751988000300005&lng=pt&nrm=iso&tlng=en doi: 10.1590/S0101-81751988000300005
- ↑ Santos R, Almeida M, Nunes J, Tinoco L, Martins, L (2003) Bromeliads as a keystone resource for the scorpion Tityus neglectus in eastern Rio Grande do Norte State. Journal of the Bromeliad Society 53: 256-258.
- ↑ Vanzolini P, Williams E (1981) The vanishing refuge: a mechanism for ecogeographic speciation. Papéis Avulsos de Zoologia 34: 251-255.
- ↑ Conceição A, Pirani J, Meirelles S (2007) Floristics, structure and soil of insular vegetation in four quatzite-sandstone outcrops of “Chapada Diamantina”, Northeast Brazil. Revista Brasileira de Botânica 30: 641–656. http://www.scielo.br/scielo.php?script=sci_abstract&pid=S0100-84042007000400009&lng=pt&nrm=iso&tlng=en doi: 10.1590/S0100-84042007000400009
- ↑ 22.0 22.1 Romero G, Vasconcelos-Neto J (2005) Spatial distribution and microhabitat preference of Psecas chapoda (Peckham & Peckham) (Araneae, Salticidae). The Journal of Arachnology 33: 124–134. http://www.americanarachnology.org/JoA_free/JoA_v33_n1/arac-033-01-0124.pdf doi: 10.1636/M03-9
- ↑ Romero G (2006) Geographic range, habitats, and host plants of bromeliad-living jumping spiders (Salticidae). Biotropica 38: 522–530. http://onlinelibrary.wiley.com/doi/10.1111/j.1744-7429.2006.00173.x/abstract doi: 10.1111/j.1744-7429.2006.00173.x
- ↑ Romero G, Vasconellos-Neto J, Trivelin P (2008). Spatial variation in the strength of mutualism between a jumping spider and a terrestrial bromeliad: Evidence from the stable isotope 15N. Acta oecologica 33: 380-386. doi: 10.1016/j.actao.2008.02.001
- ↑ Romero G, Mazzafera P, Vasconcellos-Neto J, Trivelin P (2006) Bromeliad-living spiders improve host plant nutrition and growth. Ecology 87: 803-808. doi: 10.1890/0012-9658(2006)87%5B803:BSIHPN%5D2.0.CO;2
- ↑ Figueira J, Vasconcellos-Neto J (1991) Paepalanthus, cupins e aranhas. Ciência Hoje 13: 20-26.
- ↑ Faith D (1992) Conservation evaluation and phylogenetic diversity. Biological Conservation 61: 1-10. doi: 10.1016/0006-3207(92)91201-3
- ↑ Fundação SOS Mata Atlântica , Instituto Nacional de Pesquisas Espaciais-INPE (2009) Atlas dos remanescentes florestais da Mata Atlântica Período 2005–2008. http://mapas.sosma.org.br/site_media/download/atlas%20mata%20atlantica-relatorio2005-2008.pdf [accessed 07 June 2012]
Images
|