Cubanicula
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Pellegrini2020PhytoKeys169, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Pellegrini2020PhytoKeys169">{{Citation See also the citation download page at the journal. |
Ordo: Commelinales
Familia: Haemodoraceae
Name
Cubanicula Hopper, J.E. Gut., E.J. Hickman, M. Pell. & Rhian J. Sm. gen. nov. – Wikispecies link – Pensoft Profile
Type species
Cubanicula xanthorrhizos (C. Wright ex Griseb.) Hopper et al. (≡ Xiphidium xanthorrhizon C. Wright ex Griseb.).
Diagnosis
Similar to Xiphidium Loefl. in inflorescence and floral morphology, differing due to its contracted stems, leaves congested into an apical rosette, 1–2-branched cincinni, extrorsely rimose anthers, capsules trigonous, 3-valved, with thickened and tomentose septal ridges, dry at maturity, dehiscence loculicidal, lenticellate, with coarse trichomes on margins and outer testa.
Etymology
Named for Cuba, in which the genus is narrowly endemic. The diminutive ‘icula’ is an allusion to the fact that this genus is second only to Pyrrorhiza in Haemodoraceae in its restricted geographical range.
Taxonomic history
The types of Xiphidium xanthorrizon were collected by the American botanist Charles H. Wright (1811–1885), who, between 1856–1867, ‘travelled all over Cuba with the exception of the highest mountains and tripled the number of the phanerogamous plant species known from this territory’ (Borhidi 1991[1]: 16). New taxa collected by Wright were described by Göttingen’s Professor August H.R. Grisebach (1814–1879), primarily in his Plantae Wrightianae e Cuba Orientali, published in two parts from 1860–1862. However, X. xanthorrizon was not published until 1866, in Griesebach’s Catalogus Plantarum Cebensium, in which he attributed the new species’ name to Wright.
Ascertaining Wright’s itinerary during his three periods on Cuban expeditions has been problematic: ‘[…] his travels were confined chiefly to the two ends of the island, leaving the great central portion largely unexplored. It is unfortunate that the labels on his plants, at least in most of the collections where they are to be found, bear only the inscription “Cuba” or “in Cuba orientali”.’ (Underwood 1905[2]: 291). Moreover, many of Wright’s collections made in western Cuba were irrevocably damaged in transport to the USA: ‘It appears from Wright’s correspondence that a considerable portion of his collection was lost, mainly that collected in the rich tobacco region of the western end of the island (Pinar del Rio). How extensive this loss may have been, probably cannot now be estimated, but it was certainly considerable.’ (Underwood 1905[2]: 291). The author also quotes some sentences found in Dr Gray’s Letters (2: 555) that explain the cause of the loss of these specimens: ‘April 8th [1867] It grieves my heart and will grieve yours badly when I tell you that your boxes were put under a cargo of wet sugar, which drained into them and have [sic] ruined the collection. […] As to specimens to dispose of, say only one-half or one-third of the whole mass is left fit for it… [Ever your disconsolate A. GRAY.]’ (Gray 1867 apudUnderwood 1905[2]: 291, 292).
These problems aside, Underwood (1905)[2] managed to assemble a sketch of Wright’s many Cuban itineraries through 200 letters written to Asa Gray and other sources that mentioned dates and place names. Perhaps because of a shipment earlier than the calamity referred to above by Asa Gray, Wright’s collections of Xiphidium xanthorrizon persist. Wright probably collected X. xanthorrizon when he was stationed at Retiro – ‘a finca near Taco Taco where Don Jose Blain lived’ (Underwood 1905[2]: 297), either in June–September 1863 or, more likely, in January–May 1864. This can be deduced from labels on the types that provide the dates 1860–1864 and a statement in a letter written in Havana on 28 July 1864: ‘plants boxed ready to embark’ (Underwood 1905[2]: 298).
The type location and Wright’s collection number of X. xanthorrizon is cited by Maas and Maas-van de Kamer (1993[3]: 31) as ‘Cuba. Pinar del Rio: San Cristobal, Wright 3259’. The only reference to San Cristobal cited by Underwood (1905[2]: 298) is for a letter written at Retiro on the 15 June 1866 – ‘went again to San Cristobal on the 10th’. Since San Cristobal is only 10 km ENE of Retiro on the main road to Havana, it is clearly a place that Wright would have gone through whenever visiting Retiro in the years 1863, 1864, and 1866. For example, on 19 May 1864, Wright wrote: “Made an excursion of ten days eastward and southward to La Concordia, San Leon, etc.” (Underwood 1905[2]: 298). Subsequent collections filled in knowledge of the geographical distribution of X. xanthorrizon, including an early collection from the 1860s by Jose Blain first recording the species from the northern portion of Isla de Juventud (= Isla de Pinos). The specimen (in the Field Museum) was annotated as Xiphidium floribundum Sw (= X. caeruleum), yet associated notes said (Millspaugh 1900[4]: 426): ‘[…] In Cuba this species grows only in shady situations in glens, never on the open savannas; here, however, it seeks the open plains far from shade – Blain.’ Moreover, an old handwritten slip attached to the Field Museum specimen, presumably written by Charles Wright, gave the species as X. xanthorrizon, and this is undoubtedly the identity of Blain’s specimen. It is X. xanthorrizon, not X. caeruleum, that is common on open savannahs on Isla de Juventud, a view affirmed in subsequent maps and accounts of Cuban Haemodoraceae (Maas and Maas-van de Kamer 1993[3]; Urquiola Cruz et al. 2000[5]). The species’ range has not been extended from the open pine woodlands on the white sands of Pinos del Rio Province and the Isla de Juventud, despite extensive modern collections across Cuba, such as the 20,000 sheets made by Borhidi (1991)[1] and colleagues in 1969–1970 and 1974–1976, for phytogeographic and vegetation mapping purposes. Until now, treatments of X. xanthorrizon after the original description have not challenged the generic placement of the species (León 1946[6]; Simpson 1990[7], 1998b[8]; Maas and Maas-van de Kamer 1993[3]; Urquiola Cruz et al. 2000[5]). Indeed, Simpson (1990[7]: 729) remarked, ‘Xiphidium consists of X. coeruleum [sic] and X. xanthorrhizos [sic], which differ only in minor morphological features and are likely more closely related to one another than to any other genus. However, because no definitive synapomorphy is evident for Xiphidium, its monophyly cannot be affirmed.’ Although he undertook a comprehensive examination of the morphology and anatomy of the genera of Haemodoraceae, Simpson (1990)[7] did not include both species of Xiphidium in his study in order to test the genus’ monophyly. Instead, he chose only to represent the genus by sampling X. caeruleum. An examination of seeds alone would have raised questions about the generic placement of X. xanthorrizon. Simpson (1993)[9] discovered the unusual absence of septal nectaries in both Xiphidium species and interpreted this trait as an autapomorphy for the genus associated with buzz pollination by bees, which was known for X. caeruleum (Buchmann 1980[10]), but the pollination ecology of X. xanthorrizon was not documented. Maas and Maas-van de Kamer (1993[3]: 11) speculated that ‘The differently coloured nectar guide on the three adaxial tepals of X. xanthorrizon suggest that an insect pollinator alights in a consistent orientation, forwardly directed to collect pollen from the shorter stamens, in the meantime being dusted by the largest stamen.’ Simpson (1993)[9] affirmed an observation of Maas and Maas-van de Kamer (1993)[3] that X. xanthorrizon has longitudinal anther dehiscence, whereas X. caeruleum anthers commence with nearly poricidal dehiscence, becoming longitudinal as flowers age or dry out (Buchmann 1980[10]). Such a difference echoed a number of other traits overlooked by many authors that call into question the hypothesis that X. xanthorrizon and X. caeruleum are sister taxa.
Regarding generic relationships of Xiphidium, Simpson (1998a[11]: 217) elaborated: ‘Within this superior-ovaried group [of subfamily Haemodoroideae], Wachendorfia and Barberetta are united in having a similar pollen ultrastructure (Simpson 1983[12], 1990[7]) and Schiekia and Pyrrorhiza are united in having staminodes and similarities in ovule anatomy (M.G. Simpson, 1990, unpubl.). The exact relationships of Xiphidium to these genera is unclear.’ Molecular phylogenetic analyses have yet to clarify the systematic position of Xiphidium in this clade (Hopper et al. 1999[13], 2009[14]).
Maas and Maas-van de Kamer (1993[3]: fig. 5) were the first to illustrate and compare SEM micrographs of the seeds of X. xanthorrizon and X. caeruleum, which differ significantly. Indeed, seeds of X. xanthorrizon resemble those of Pyrrorhiza in being large (i.e., 2.5–3.5 mm long) and covered with 1–1.5 mm long coarse hairs (Fig. 4D, H), whereas X. caeruleum has cuboid, black seeds 0.5–1.0 mm in diameter and they are minutely tuberculate, lacking hairs (Fig. 4L), similar to seeds of Schiekia (i.e., S. orinocensis and S. timida). Maas and Maas-van de Kamer (1993[3]: 10) suggested that ‘the hairy seeds of Xiphidium xanthorrhizon and Pyrrorhiza neblinae, both savanna plants, might very well be dispersed by animals having seeds adhering to their body (i.e., exozoochoric dispersal).’ Simpson (1990[7]: 754) scored X. caeruleum as enantiostylous, but with ‘actinomorphic and erect (not zygomorphic and horizontal) flowers without any bilaterally symmetric nectar guides.’ Maas and Maas-van de Kamer (1993[3]: 11) affirmed that X. xanthorrizon ‘clearly displays’ enantiostyly of the latter kind, differing significantly from the flowers of X. caeruleum. Despite these floral differences and significantly divergent seed morphologies between X. xanthorrizon and X. caeruleum, these authors retained the traditional circumscription of Xiphidium s.lat. With the recognition of a second species of Xiphidium s.str. in the present study, it became clear that the inclusion of X. xanthorrizon in Xiphidium s.lat. was untenable from the morphological perspective (Pellegrini 2019[15]), added to strong molecular support (Hopper et al. in prep).
Comments
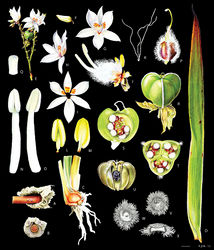
Cubanicula is recovered with strong bootstrap support in a clade with Xiphidium s.str. and Pyrrorhiza Maguire & Wurdack, sister to the latter genus, not Xiphidium, in which the species of Cubanicula was initially placed (Hopper et al., in prep). This clade can be morphologically supported by the presence of sand-binding roots, campanulate and pollen rewarding flowers, tepals with an apical black mucron, anthers as long as to ca. ½ times shorter than the filaments, vestigial or completely lacking septal nectaries, crateriform stigmas, and enlarged placental attachments subtending the ovules (Hickman 2019[16]; Pellegrini 2019[15]). Cubanicula can be differentiated from Pyrrorhiza by its rhizomatous underground system (vs. cormose in Pyrrorhiza), thyrsi 1–2-branched cincinni (vs. always unbranched), flower enantiostylous (vs. non-enantiostylous), upper tepals with three orange-yellow to orange nectar guides (vs. lacking nectar guides), stamens 3 (vs. one), lateral filaments twisted and medial filament bent upwards (vs. lateral stamens staminodial and medial filament straight) and staminodes absent (vs. staminodes 2, filiform). The difference between Cubanicula and Xiphidium s.str. is especially evident in capsule and seed characters, as well as floral size. These genera can be differentiated by the characters summarised in Table 1 and the fruit and seeds characters illustrated in Fig. 4.
| Character | Cubanicula | Xiphidium s.str. |
|---|---|---|
| Stems | Contracted | Elongated |
| Leaves | Congested at the apex of the stems forming a rosette | Evenly distributed along the stems |
| Cincinni | 1–2-branched | Unbranched |
| Flowers | Large, bicoloured | Small, uniformly coloured, rarely bicoloured |
| Stamens | Dimorphic, anthers extrorsely rimose, anther sacs asymmetric | Monomorphic, anthers introrsely rimose, but functionally poricidal, anther sacs symmetric |
| Enlarged placental attachment | Capitate, vertically compressed, red | Cylindrical, truncate, green |
| Capsules | Trigonous, loculicidal 3-valved, dry at maturity, septal ridges tomentose at maturity | Subglobose to globose, indehiscent, somewhat fleshy at maturity, septal ridges glabrous at maturity |
| Seeds | Lenticellate | Cuboid |
| Testa | Coarse trichomes on margins and outer surface, glabrous on hilar surface | Tuberculate |
Original Description
- Pellegrini, M; Hickman, E; Guttiérrez, J; Smith, R; Hopper, S; 2020: Revisiting the taxonomy of the Neotropical Haemodoraceae (Commelinales) PhytoKeys, 169: 1-59. doi
Images
|
Other References
- ↑ 1.0 1.1 Borhidi A (1991) Phytogeography and Vegetation Ecology of Cuba. AkadCmi Kiado Publishing House, The Hungarian Academy of Sciences.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Underwood L (1905) A summary of Charles Wright’s explorations in Cuba.Bulletin of the Torrey Botanical Club32(6): 291–300. https://doi.org/10.2307/2478811
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Maas P, Maas-van de Kamer H (1993) Haemodoraceae. Fl.Neotropica61: 1–44.
- ↑ Millspaugh C (1900) Plantæ Utowanæ. Plants collected in Bermuda, Porto Rico, St. Thomas, Culebras, Santo Domingo, Jamaica, Cuba, The Caymans, Cozumel, Yucatan, and the Alacran Shoals. Dec. 1898 to Mar. 1899. The Antillean cruise of the Yacht Utowana. Mr. Allison V. Armour, Owner and Master, part I – Catalogue of the Species.Publications of the Field Columbian Museum, Botanical Series2: 3–110. https://www.biodiversitylibrary.org/page/46063084
- ↑ 5.0 5.1 Urquiola Cruz A, Aguilar Trujillo J, Betancurt Betancurt Z, Betancurt Gandul M (2000) Haemodoraceae. In: Greuter W (Ed.) Flora de la República de Cuba. Series A. Plantas Vasculares.Fascículo 5(2), Koeltz Scientific Books, Königst, 2 pp.
- ↑ León H (1946) Flora de Cuba (Vol. 1). Gymnosperms Monocotyledons. Contribuciones Ocasionales del Museo de Historia Natural Colegio La Salle 8, Cultural S.A., La Habana.
- ↑ 7.0 7.1 7.2 7.3 7.4 Simpson M (1990) Phylogeny and classification of the Haemodoraceae.Annals of the Missouri Botanical Garden77(4): 722–784. [pl. XXI–XXIII.] https://doi.org/10.2307/2399670
- ↑ Simpson M (1998b) Haemodoraceae. In: Kubitzki K (Ed.) The Families and Genera of Vascular Plants (Vol.4). Springer Verlag, Berlin, 212–128.
- ↑ 9.0 9.1 Simpson M (1993) Septal nectary anatomy and phylogeny in the Haemodoraceae.Systematic Botany18(4): 593–613. https://doi.org/10.2307/2419536
- ↑ 10.0 10.1 Buchmann S (1980) Preliminary anthecological observations on Xiphidium caeruleum Aubl. (Monocotyledoneae: Haemodoraceae).Panama Journal of the Kansas Entomological Society53(4): 685–699.
- ↑ Simpson M (1998a) Reversal in ovary position from inferior to superior in the Haemodoraceae: Evidence from floral ontogeny.International Journal of Plant Sciences159(3): 466–479. https://doi.org/10.1086/297564
- ↑ Simpson M (1983) Pollen ultrastructure of the Haemodoraceae and its taxonomic significance.Grana22(2): 79–103. https://doi.org/10.1080/00173138309431969
- ↑ Hopper S, Fay M, Rossetto M, Chase M (1999) A molecular phylogenetic analysis of the bloodroot and kangaroo paw family, Haemodoraceae: Taxonomic, biogeographic and conservation implications.Botanical Journal of the Linnean Society131(3): 285–299. https://doi.org/10.1111/j.1095-8339.1999.tb00770.x
- ↑ Hopper S, Smith R, Fay M, Manning J, Chase M (2009) Molecular phylogenetics of Haemodoraceae in the Greater Cape and Southwest Australian Floristic Regions.Molecular Phylogenetics and Evolution51(1): 19–30. https://doi.org/10.1016/j.ympev.2008.11.015
- ↑ 15.0 15.1 Pellegrini M (2019) Systematics of Commelinales focusing on Neotropical lineages. PhD thesis. Universidade de São Paulo, São Paulo, SP, Brazil.
- ↑ Hickman E (2019) Discovery through illustration – botanical art, traits and their phylogeny in the Haemodoraceae. PhD thesis, The University of Western Australia.