Asthenopus curtus
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Molineri2015ZooKeys, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Molineri2015ZooKeys">{{Citation See also the citation download page at the journal. |
Ordo: Ephemeroptera
Familia: Polymitarcyidae
Genus: Asthenopus
Name
Asthenopus curtus (Hagen) – Wikispecies link – Pensoft Profile
- Palingenia albifilum var.; Walker 1853[1]: 554.
- Palingenia curta Hagen 1861[2]: 304.
- Campsurus curtus; Eaton 1868[3]: 84; Eaton 1883[4]: 40; Ulmer 1921[5]: 240.
- Asthenopus curtus; Eaton 1871[6]: 59; Ulmer 1920[7]c: 107; Ulmer 1921[5]: 240; Lestage 1922[8]b: 142; Ulmer 1942[9]: 105; Traver 1956[10]b: 7; Kimmins 1960[11]: 312; Sattler 1967[12]: 104; Berner 1978[13]: 103; Hubbard 1982[14]a: 270; Domínguez 1988[15]a: 24; Hubbard and Domínguez 1988[16]: 207; Domínguez 1989[17]a: 173 (described as Asthenopus magnus sp. n. below); Domínguez et al. 2006[18]: 561.
- Campsurus amazonicus Hagen 1888[19]: 230.
- Asthenopus amazonicus; Ulmer 1920[7]c: 107; Lestage 1923[20]: 124; Ulmer 1942[9]: 106; Traver 1950[21]: 606; Traver 1956[10]b: 7; Sattler 1967[12]: 104; Berner 1978[13]: 103.
Type material
Photographs of the type at the British Museum were studied.
Additional material. Two male imagos (IBN, slide 480) from COLOMBIA, Amazonas, Leticia, caño km 15, S 4°5'41" − W 69°59'1", 93 m, 11.ii.1999, light trap 4−6 h, E. Domínguez, M.C. Zúñiga & C. Molineri cols.; male imaginal slides (FAMU) from BRAZIL, Amazonas, Careiro Island, Divinopolis, SE of Manaus, 29.vii.1961, E.J. Fittkau; 2 male and 1 female pharate subimagos (IBN642CM-eggs, 643-female, 644-male) from BRAZIL, Amazonas, São Paulo de Olivença, Bom Sucesso, 4.ix.2003, (aprox. S 3°28' − W 68°59').
Diagnosis
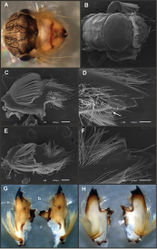
Asthenopus curtus is the type species of the genus, and is known from adults of both sexes, nymphs and eggs. Nine autapomorphies were recovered in the cladistic analysis, and are useful to diagnose the species (see Appendix 2). The following combination of characters is useful to distinguish Asthenopus curtus from the other species of the genus: 1) male FW 10.0, female FW 14.0–18.5; 2) male foreleg length 0.69–0.74 times the length of FW; 3) pronotum width/length ratio: 2.0–2.3 (male), 2.7–3.0 (female); 4) 18–25 marginal intercalary veins present on the entire margin of forewings (Fig. 16A–B), 2–3 times shorter than distance between longitudinal veins in male (not anastomosed), hind wings with marginal intercalaries in at least 4 spaces between main veins; 5) male FW with 0 to 1 crossveins between Rs and MA basal to Rs fork; 6) ratio total length/basal width of forceps 5.4 (Fig. 17A–B); 7) penes very sclerotized, contrasting strongly with the remaining genital parts, apex projecting acutely; a deep furrow separates penis lobe from thumb, median remnant of styliger plate subrectangular without marked projections, pedestals subrectangular and large, outer margin projecting posteriorly on outer margin along forceps base; 8) female sternum VIII with anteromedian keel and reduced sockets as in Fig. 18G; 9) egg ratio maximum width of egg/maximum width of PC 1.1–1.3, cap formed by 3–5 filaments, chorionic plates separated by smooth chorion (Fig. 18A); 10) nymphal mandible: ratio total length of mandible/mandibular tusk length 1.6–1.7; 11) space between the subbasal and the submedian tubercles in inner margin of left mandibular tusks is short and concave (Fig. 14A).
Male imago. Length: body, 8.0–8.7; FW, 10.0; HW, 4.4; foreleg, 7.5; cerci, 33.0–35.0. General coloration yellowish light brown. Head whitish, heavily shaded black dorsally, paler on posteromedian zone of occiput, black shading extending anteriorly on frons as two parallel lines surrounding median ocellus. Antennae pale, slightly shaded gray on dorsum. Thorax. Pronotum yellowish translucent completely shaded gray, darker on anterior ring; paler on two transverse lines, one separating anterior and posterior rings and another more posterior and obliquely transverse; pleurae shaded with black, sternum with a median gray macula. Pronotum width/length ratio: 2.0–2.3. Mesonotum whitish yellow (or brownish in some males) with a black median triangle between posteroscutal protuberances, metanotum similar in color, also shaded black posteromedially; mesopleurae and sterna paler, shaded with black along anterior margin of katepisternum. Legs yellowish white shaded with gray dorsally on all coxae, femora and tibiae; foretarsal segment 1 blackish (Fig. 20G), remaining tarsal segments paler shaded with gray distally, claws grayish thin troughout. Wings (Fig. 16A–B). Membrane hyaline shaded very slightly with brownish near anterior margin and turning whitish translucent towards apical zone of C–Sc areas; veins translucent shaded with brown. Abdomen yellowish white shaded extensively with grayish brown dorsally, darkening very slightly towards rear segments. Sterna pale very slightly shaded gray, shaded stronger on mediolongitudinal line near anterior margin of sterna VIII–IX, this line is blurred posteriorly; a grayish black triangular mark is present at each side of this line, on anterior margin of sterna VIII–IX; sternum X shaded black except medially. Genitalia (Fig. 17A–B): median remnant of styliger plate and pedestals yellowish, forceps whitish translucent shaded gray along outer margin, penes dark orange with whitish base. Caudal filaments whitish, shaded gray at base of terminal filament.
Female adult. Length: body, 10.5–13.2; FW, 14.0–18.5; HW, 5.7; cerci, 5.8–7.0. Morphologically very similar to female adults of Asthenopus angelae described in detail in de Souza and Molineri (2012)[22]. Here only those characters that differ from the cited description are mentioned. Pronotum almost 3 times wider than long, width/length ratio = 2.7–3.0 (see continuous characters in phylogenetic matrix, Appendix 3). Mesonotum uniformely brownish (cuticular pigmentation), almost without gray markings (dermic pigments). Female sternum VIII with anteromedian keel and reduced sockets as in Fig. 18G. Cercus about half the length of FW, cercus length/FW length: 0.4–0.5.
Eggs (Fig. 18A). Length, 200–220 µ; width, 130–155 µ. Two polar caps (maximum width, 110–120 µ), formed by 3–5 very long coiled threads. Chorionic surface smooth with relatively large subcircular chorionic plates, the plates are regularly spaced and some of them are divided in two or three subequal parts.
Mature nymph. Length of male: body, 9.5–9.7; cercus, 7.0; terminal filament, 5.0. Length of female: body, 17.0; cercus, 8.0; terminal filament, 7.0. Only characters that differ from Asthenopus angelae are given here, refer to that description for more detailed information. Head (occipital area) dorsally brownish uniformly shaded with gray. Mouthparts. Left mandibular tusks with a relatively shorter space between the large subbasal tubercle and the smaller subdmedian one, this space is somewhat C-shaped (Fig. 14A). Right mandible with distal corner of mola strongly protruding. Thorax. Mesonotum uniformly brownish (cuticular) without strongly gray-shading on carinae (Fig. 4E). Legs and paraprocts identical to those on Fig. 15.
Distribution
Amazonas River from Leticia (Colombia) to Manaus (Brazil).
Discussion
Much confusion exists in the literature concerning this species. Many authors mention Asthenopus curtus but from missidentified material. For example Ulmer (1942)[9] described and illustrated (as Asthenopus curtus) a pair of males of Asthenopus angelae. The material from Ecuador studied by Domínguez (1988)[15] proved to be a different but related species (Asthenopus magnus sp. n.). Berner (1978)[13] synonymized Asthenopus curtus with Asthenopus amazonicus, showing that the differences between both species were only attributable to sexual dimorphism, but he was working with Asthenopus angelae males (de Souza and Molineri 2012[22]). Nevertheless, Berner conclusions were correct given that sexual dimorphism in FW venation is present in both species. As it is impossible to assign any specimen to Asthenopus amazonicus, we prefer to treat it as synonym of Asthenopus curtus, as Berner proposed. Actually, only one specimen from previous works is positively determined as Asthenopus curtus: the type, studied by Eaton (1883)[4] and illustrated by Kimmins (1966). We add here some other records from the Amazonas River: a pair of males from Colombia, some reared nymphs from Brazil and Fittkau’s slides at FAMU. These male imagos show the characteristic genitalia of the holotype of Asthenopus curtus, with extremely wide forceps, long penis lobes and slender and very acute apical spines, and the more or less uniform brownish mesothoracic coloration (an exception of this last character are the males from Colombia-Leticia, much paler). The egg of Asthenopus curtus (Fig. 18A) is similar to that of Asthenopus hubbardi (Fig. 18D), in the shape and relative large size of the disk-like structures that leaves exposed only a reduced surface of smooth chorion. On the contrary the egg of Asthenopus angelae presents smaller disk-like structures with a larger surface of smooth chorion among them (Fig. 18B).
As the result of the present study, the female adult, egg, and nymphal stages are described here for the first time. Previous descriptions of female and nymphs in the literature were done from specimens of Asthenopus angelae or other species but are not useful to clearly distinguish the species.
Taxon Treatment
- Molineri, C; Salles, F; Peters, J; 2015: Phylogeny and biogeography of Asthenopodinae with a revision of Asthenopus, reinstatement of Asthenopodes, and the description of the new genera Hubbardipes and Priasthenopus (Ephemeroptera, Polymitarcyidae) ZooKeys, (478): 45-128. doi
Images
|
Other References
- ↑ Walker F (1853) Ephemeridae. List of the specimens of neuropterous insects in the collection of the British Museum. The Trustees, London, 533–585.
- ↑ Hagen H (1861) Synopsis of the Neuroptera of North America with a list of the South American species. Smithsonian Miscellaneous collections, 1–347.
- ↑ Eaton A (1868) An outline of re-arrangement of the genera of Ephemeridae. Entomologist Monthly Magazine 5: 82–91.
- ↑ 4.0 4.1 Eaton A (1883–1888) A revisional monograph of recent Ephemeridae or mayflies. Transactions of the Linnean Society of London, 2nd Ser Zoology 2: 1–352.
- ↑ 5.0 5.1 Ulmer G (1921) Über einige Ephemeropteren-Typen älterer Autoren. Archiv für Naturgeschichte, Abteilung A 87: 229–267.
- ↑ Eaton A (1871) A Monograph of the Ephemeridae. Transactions of the Entomological Society of London 1871: 1–164.
- ↑ 7.0 7.1 Ulmer G (1920) Übersicht über die Gattungen der Ephemeropteren, nebst Bemerkungen über einzelne Arten. Stettiner Entomologische Zeitung 81: 97–144.
- ↑ Lestage J (1922) Notes sur les genres Asthenopus-Povilla (Ephemeroptera) et description d’une espèce javanaise nouvelle (Asthenopus corporaali sp. n.). Annales de la Société entomologique de Belgique 62: 142–148.
- ↑ 9.0 9.1 9.2 Ulmer G (1942) Alte und neue Eintagsfliegen (Ephemeropteren) aus Süd- und Mittelamerika. Stettiner Entomologische Zeitung 103: 98–128.
- ↑ 10.0 10.1 Traver J (1956) The genus Asthenopodes (Ephemeroptera). Comunicaciones zoologicas del Museo de Historia Natural de Montevideo 4: 1–10.
- ↑ Kimmins D (1960) The Ephemeroptera types of species described by A.E. Eaton, R. McLachlan and F. Walker, with particular reference to those in the British Museum (Natural History). Bulletin of the British Museum (Natural History) Entomology 9: 269–318.
- ↑ 12.0 12.1 Sattler W (1967) Über die Lebensweise, insbesondere das Bauverhalten, neotropischer Eintagsfliegen-Larven (Ephemeroptera, Polymitarcidae). Beiträge zur Neotropischen Fauna 5: 89–110. doi: 10.1080/01650526709360399
- ↑ 13.0 13.1 13.2 Berner L (1978) The status of Asthenopus curtus (Hagen) (Ephemeroptera: Polymitarcyidae). Acta Amazonica 8: 103–105.
- ↑ Hubbard M (1982) Catalogo abreviado de Ephemeroptera da America do sul. Papeis Avulsos de Zoologia, Sao Paulo 34: 257–282.
- ↑ 15.0 15.1 Domínguez E (1988) Asthenopus gilliesi sp. n. y su importancia en la taxonomía de la subfamilia Asthenopodinae (Ephemeroptera: Polymitarcyidae). Anales del Museo de Historia Natural de Valparaíso 19: 21–26.
- ↑ Hubbard M, Dominguez E (1988) Synonymy of the neotropical mayfly genera Asthenopus and Asthenopodes (Ephemeroptera : Polymitarcyidae : Asthenopodinae). The Florida Entomologist 71: 207–210. doi: 10.2307/3495369
- ↑ Domínguez E (1989) Primera cita de Asthenopus curtus (Hagen) (Ephemeroptera : Polymitarcyidae) para la Republica de Ecuador. Revista de la Societad Entomologica Argentina 45: 173–174.
- ↑ Domínguez E, Molineri C, Pescador M, Hubbard M, Nieto C (2006) Ephemeroptera of South America. Aquatic Biodiversity in Latin America. Pensoft, 650 pp.
- ↑ Hagen H (1888) Unsere gegenwärtige Kenntniss der Ephemeren. Entomologische Zeitung Stettin 49: 221–232.
- ↑ Lestage J (1923) L’imbroglio campsurien. Notes critiques sur les Campsurus (Ephemeroptera). Annales de la Société entomologique de Belgique 63: 113–124.
- ↑ Traver J (1950) Notes on Neotropical Mayflies. Part IV. Family Ephemeridae (continued). Revista de Entomologia 21: 593–614.
- ↑ 22.0 22.1 De-Souza M, Molineri C (2012) The adults and nymphs of Asthenopus angelae new species (Ephemeroptera: Polymitarcyidae) from Argentina, Bolivia, Brazil and Colombia. Zootaxa 3399: 45–52.