Asthenopodes
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Molineri2015ZooKeys, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Molineri2015ZooKeys">{{Citation See also the citation download page at the journal. |
Ordo: Ephemeroptera
Familia: Polymitarcyidae
Name
Asthenopodes Molineri & Salles & Peters, 2015 stat. n. – Wikispecies link – Pensoft Profile
- Asthenopodes Ulmer 1924[1]: 26; Traver 1950[2]: 611; Traver 1956[3]: 1; Hubbard 1975[4]: 111; Hubbard and Domínguez 1988[5]: 209; Domínguez 1988[6]: 24.
Type species
Palingenia albicans Pictet, original designation (= Asthenopodes picteti Hubbard)
Species included
Asthenopodes picteti Hubbard, Asthenopodes traverae sp. n., Asthenopodes chumuco sp. n.
Diagnosis
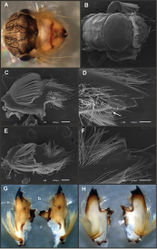
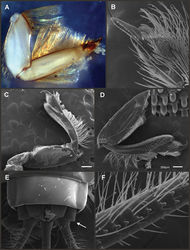
Seven autapomorphies define the genus Asthenopodes in our cladistic analysis (Appendix 2), some of them include: 1) Male foretarsal segment 1 fused with tibia (Figs 20A–C); 2) apex of male foretarsal claw strongly expanded (apex 3 times wider than stalk, Fig. 13E–F); and 3) pedestal relatively large, elongated, narrow at the base (Fig. 12). The following combination of characters representing the entire range of variation of the three included species, is useful to distinguish Asthenopodes from other genera in Polymitarcyidae: 1) ratio length male FW/foreleg = 1.0–1.6; 2) tarsal segment 1 indistinct (fused to tibia), tibia distally subequal in width to base of tarsal segment 2 (Figs 20A–C); 3) pronotum width/length ratio: 1.2–1.9 (male), 1.5–2.3 (female); 4) in both sexes FW marginal intercalary veins relatively long and anastomosed (from 9 to 22 in male FW, Fig. 11); 5) in both sexes FW with 3–6 (most commonly 4, but variable depending in size of specimen) crossveins between R and M, basally to R fork; 6) FW vein IMP slightly longer than MP1, both frequently free at base but may be joined to one another and to MP2 by crossveins (Fig. 11); 7) median remnant of styliger plate present in Asthenopodes chumuco (subrectangular as other Asthenopodinae) but medially very short and with a strongly marked lateral lobe in the sister pair Asthenopus picteti-Asthenopodes traverae; pedestals relatively large and thinner at the base; 8) forceps relatively slender, ratio length/basal-width = 4.7–9.5; 9) penes variable in form but curved medially rather than ventrally, basal thumb well separated from penial lobe, penial lobe apically acute (Fig. 12); 10) female abdominal sternum VIII with anteromedian paired sockets reduced in size (Fig. 13D); 11) eggs (Fig. 13A–C) with relatively small polar caps subequal to much thinner than egg, formed by 5–16 threads, chorion covered with medium-sized circular plates, surrounded by many smaller plates; 12) nymphal head dorsally strongly convex on occiput, frons not projecting medially (Figs 9A–B); 13) nymphs with very short and robust tusks (Figs 9C–H), without large submedian inner tubercle, with 2 or 3-pointed apex (asymmetric); 14) nymphal foretarsal claw with 2 rows of marginal denticles, a long row of 15 denticle and one shorter row of 12 denticles (Fig. 10B); 15) apex of femur dorsally with ca. 30 strong and rounded spines (Fig. 10D).
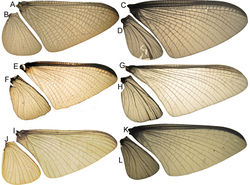
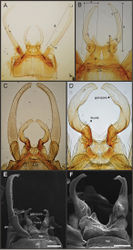
Male imago. Length (mm): body, 7.3–13.5; FW, 7.0–14.5; HW, 3.7–7.3; foreleg, 5.0–14; cerci, 20.0–38.5. Antennae: scape subequal to pedicel; flagellum bristle-like. Thorax. Pronotum width/length: 1.2–1.9. Legs. Forelegs relatively long, ratio length FW/foreleg = 1.0–1.6; tarsal segment 1 fused to tibiae (Fig. 20A–C); longest segment is tarsal segment 2 or tibia (variable), ratio length tarsal segment 2/tibia = 0.6–1.5; tarsal segments long decreasing in length from 2>3>4>5; claws different in size, one long the other short, strongly widened distally (Fig. 13E–F). Wings (Fig. 11). FW with 9–22 marginal intercalaries along hind margin, also present along entire hind margin of HW; these intercalaries present numerous connections with other cross and longitudinal veins (i.e., very anastomosed); 3–6 crossveins between R and M sectors basally to R fork; Rs stem length/Rs from fork to margin = 0.2–0.4; ratio MA from fork to margin/stem length = 9–15; IMP fused basally to MP1 or free; MP2 fused to IMP. Genitalia (Fig. 12): median remnant of styliger plate with posterolateral corners roundly projecting, pedestal long to very long; forceps relatively long and slender, ratio length/basal-width = 4.7–6.7. Terminal filament reduced, cerci long (ratio length FW/cercus = 0.32–0.44).
Female length (mm): body, 7.2–19.0; FW, 12.2–22.5; HW, 5.3–11.5; cerci 1.2–4.0. Thorax. Pronotum width/length = 1.5–2.3. Wings with crossveins and intercalaries more numerous than in male. Abdominal sternum VIII (Fig. 13D) with reduced paired anteromedian sockets, sockets small, shallow and contiguous located at the base of a blunt subquadrate projection. Terminal filament reduced, shorter than tergum VIII, with few thin annuli; cercus very short, 0.1–0.2 times the length of FW.
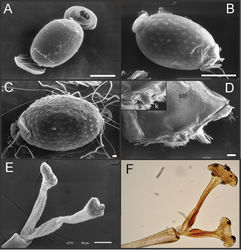
Eggs (Fig. 13A–C). Length, 221–355 μ; width, 143–240 μ. Oval (ratio maximum length / maximum width = 1.3–1.7), with two relatively small polar caps on apices (ratio maximum with of egg/maximum width of uncoiled polar cap = 1.2–3.1), each formed by 5–16 long coiled filaments. Chorionic surface with fine granulated aspect or regularly spaced plates.
Nymphs, nearly mature (Fig. 4B, F). Length (mm): body, 7.8; cerci, 2.0–2.3; terminal filament, 3.1. Head subquadrate in dorsal view, smooth (without pilose areas), antennae subequal in length to head. Occipital region well developed, convex (Figs 9A–B). Head capsule dorsally projecting at bases of antennae. Frontal ridge marked only by a dense transversal row of setae; frons not projecting medially; clypeus and labrum membranous and small, labrum densely covered with long setae on dorsum. Mandibular tusks very short and robust, the part visible in dorsal view ca. 1/3 the length of head capsule; left tusk (Fig. 9E–H) apically with 3 teeth, the median reduced in length, and the innermost is strongly widened appearing as a ridge with two points (Fig. 9F); right tusk (Fig. 9C–D, G–H) with 2 distal teeth; inner surface with a small tubercles located distally (in relation to other Asthenopodinae), dorsal surface with a small tubercle (“b” in Fig. 9G) that forms an additional articulation between mandible and head capsule; tusk densely covered with long setae, except at apex. Body of mandible: molae strongly protruded medially, canines present but small, margin between them sharp-edged (acutely protruding in right mandible); with basal U-row of long filtering setae in both mandibles. Thorax. Pronotum with anterior ring (collar) subequal in length to posterior ring (length taken at the medio-longitudinal line), anterolateral corners projecting, spine-like, posterior ring with dense patches of short setae medially (Figs 4F, 9B). Legs (Fig. 10A–D). Leg I (Fig. 10A): femora robust, relatively slender, with a U-shaped ventro-basal row of long filtering setae, distal points of the U almost touching each other; tibio-tarsus (fused) with 2 U-rows of filtering setae: 1 on anterior face (each branch well separated in the base) and 1 on inner margin, with the branches near each other, apex of tibio-tarsus relatively pointed; tarsal claw with 2 rows of marginal denticles (Fig. 10B). Leg II (Fig. 10C): smaller, with thinner femora, with scattered long setae basally and a row of long and short setae along outer margin; tibia and tarsi with row of long setae on outer (dorsal) margin, ventrally with many stout spines on apical half, anterior face of tibia with a distal row of thick setae (at base of tarsus) and with a crown of thick setae at apex; tarsal claw weaker, without denticles. Leg III (Fig. 10D): intermediate in size, outer margin of femur with row of short setae, longer at apex, distal corner of femur densely covered with thick, blunt setae; inner margin of femur, tibia and tarsus densely covered with short setae; margin between tibia and tarsus with row of thick setae; outer margin of tibia and tarsus with row of long pectinated setae. Coxae I and II directed ventrally, coxa III directed postero-laterally. Abdomen. Sternite I longer than the others and partially fused with metasternum. Gill I reduced in size, whitish, single and lanceolate. Gills II–VII well developed, ventral portion smaller than dorsal portion. Tergum X without posterolateral spine (Fig. 10E). Cerci slightly shorter than terminal filament, with long setae at joinings, basal 1/4 with thick blunt setae ventraly (Fig. 10F).
Distribution
Amazonas and Parana subregions (Argentina, Brazil, Colombia, Guyana, Uruguay).
Discussion
Asthenopodes and Asthenopus have been treated as synonyms (Hubbard and Domínguez 1988[5]) after the discovery of Priasthenopus gilliesi (Domínguez, 1988), that somewhat blurred the distinction between both genera. Additionally, Hubbard and Domínguez (1988)[5] based their synonymic proposal in the fact that all known nymphs of Asthenopodinae from South America where indistinguishable and could be classified in a single genus. As the knowledge of this group had largely improved in last years we are proposing here a new rearrengement of supraspecific taxa.
The characteristics traditionally associated with Asthenopodes (summarized in Domínguez 1988[6]) are: 1) ratio foreleg/forewing of male: 7/8; 2) male foretarsus 3.5 times longer than foretibia; 3) foretarsal segment 2 very long (almost as long as tarsal segments 3 and 4 combined, and 1.5 times the length of tibia); 4) Rs fork base to margin: 2.5/10; 5) cubital intercalaries parallel, ICu2 arising basally from ICu1, or basally free but connected to ICu1 and CuP by cross veins, 6) ICu2 ending at hind margin, 7) long marginal intercalary veins present; 8) forceps ratio width/length: 1/10; 9) penes thin from the base; 10) MA fork base to margin 7/100; 11) IMP–MP1 fused; 12) MP2-IMP similar in length, fused; 13) foretarsal claws of male greatly expanded apically. The discovery of Priasthenopus gilliesi (Domínguez) put in doubt the value of characters 5, 7, 8, 9 and 10, for generic diagnoses, because this species showed some intermediary states between Asthenopodes and Asthenopus (Domínguez 1988[6], Hubbard and Domínguez 1988[5]). In our phylogenetic analysis the intermediate position of Priasthenopus gilliesi is mantained, but it is clearly located outside both genera.
The revalidation of the genus Asthenopodes Ulmer is based not only in the clade that its type species (Asthenopodes picteti) forms with other two new species, but also on the fact that the nymph shows characters considered important at the generic level in the family, mainly the shape of nymphal mandibular tusks, legs and gill I.
Key to the species of Asthenopodes
Male imagos
Female and eggsTaxon Treatment
- Molineri, C; Salles, F; Peters, J; 2015: Phylogeny and biogeography of Asthenopodinae with a revision of Asthenopus, reinstatement of Asthenopodes, and the description of the new genera Hubbardipes and Priasthenopus (Ephemeroptera, Polymitarcyidae) ZooKeys, (478): 45-128. doi
Images
|
Other References
- ↑ Ulmer G (1924) Einige alte und neue Ephemeropteren. Konowia 3: 23–37.
- ↑ Traver J (1950) Notes on Neotropical Mayflies. Part IV. Family Ephemeridae (continued). Revista de Entomologia 21: 593–614.
- ↑ Traver J (1956) The genus Asthenopodes (Ephemeroptera). Comunicaciones zoologicas del Museo de Historia Natural de Montevideo 4: 1–10.
- ↑ Hubbard M (1975) The genus Asthenopodes Ulmer and its type species (Ephemeroptera: Polymitarcidae). The Florida Entomologist 58: 111–112. doi: 10.2307/3493392
- ↑ 5.0 5.1 5.2 5.3 Hubbard M, Dominguez E (1988) Synonymy of the neotropical mayfly genera Asthenopus and Asthenopodes (Ephemeroptera : Polymitarcyidae : Asthenopodinae). The Florida Entomologist 71: 207–210. doi: 10.2307/3495369
- ↑ 6.0 6.1 6.2 Domínguez E (1988) Asthenopus gilliesi sp. n. y su importancia en la taxonomía de la subfamilia Asthenopodinae (Ephemeroptera: Polymitarcyidae). Anales del Museo de Historia Natural de Valparaíso 19: 21–26.