Macrosaccus robiniella
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Davis2011ZooKeys98, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Davis2011ZooKeys98">{{Citation See also the citation download page at the journal. |
Ordo: Lepidoptera
Familia: Gracillariidae
Genus: Macrosaccus
Name
Macrosaccus robiniella (Clemens) comb. n. – Wikispecies link – Pensoft Profile
- Lithocolletis robiniella Clemens 1859[1] (Nov.): 318; 1872[2]: 66.- Chambers 1871[3]: 54, 87, 163, 183, 185; 1872[4]: 9, 107; 1875[5]: 228; 1877[6]: 137.- Zeller 1875[7]: 348.- Frey and Boll 1878[8]: 275.- Riley 1891[9]: 109, No. 5889.- Busck 1903[10]: 189.- Dyar 1902 [1903][11]: 551, No 6267.- Braun 1908[12]: 291.- Meyrick 1912a[13]: 7; 1912b[14]: 32.- Braun 1914[15]: 110.- Forbes 1923[16]: 192.- McDunnough 1939[17]: 95, No. 9191.- Weaver and Dorsey 1965[18]: 934; 1967[19]: 178.
- Phyllonorycter robiniella (Clemens).- Ely 1918[20]: 59.- Davis 1983[21]: 10.- Maier and Davis 1989[22]: 15.- Leraut 1997[23]: 96.- De Prins and De Prins 2005[24]: 342.- De Prins and De Prins 2011[25].
- Argyromiges pseudacaciella Fitch 1859[26]: 836, No. 335.
- Lithocolletis pseudacaciella (Fitch).- Riley, 1891: 109, No. 5889 (synonym of Lithocolletis robiniella).- Dyar 1902 [1903][11]: 551, No 6267.- Braun 1908[12]: 291.- Meyrick 1912a[13]: 7; 1912b[14]: 32.- Ely 1918[20]: 59.- Barnes and McDunnough 1917[27]: 187, No. 7915.- McDunnough 1939[17]: 95, No. 9191.- Davis 1983[21]: 10.- Leraut 1997[23]: 96.- De Prins and De Prins 2005[24]: 342.- De Prins and De Prins 2011[25].
Diagnosis
The overall appearance of this widespread eastern North American (and now well established European) species most closely resembles that of the more southwestern US species, Macrosaccus neomexicanus. The more abruptly constricted apical region of the valvae and the minute, longitudinally oriented striae and spicules of the corpus bursae readily distinguish it from Macrosaccus neomexicanus.
Adult
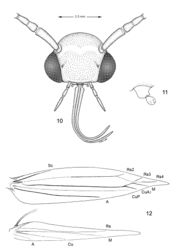
(Figs 2–4). Forewing length 2.3–3.1 mm.
Head: Frons smooth, shiny white. Vertex extremely rough; vestiture consisting of a tuft of elongate, piliform, mostly dark brown, intermixed with white, scales. Labial palpus white. Antenna mostly dark fuscous dorsally for most its length, with dark area narrowing to a more slender dark streak toward basal 1/4–1/3 its length; antenna mostly white ventrally; apical segment entirely white.
Thorax: Dark brown dorsally, white ventrally; tegula dark brown, with pale grey to white suffusion anteriorly. Forewing pattern complex, costal half mostly light orange brown crossed by 4 equally spaced, white costal strigulae, each bordered basally, sometimes faintly, by black to dark grey and distally by light grey scales; basal 2 strigulae strongly oblique; a fifth, minute, white strigula sometimes arising from black apical spot before forewing apex. Basal third and dorsal half of forewing usually darker, mostly black to sometimes pale golden grey between strigulae; slender white streak from base of wing usually indistinct or absent; a greyish, oblique strigula often evident near base of wing which connects with a larger, more distinct greyish strigula from dorsal margin; dorsal margin also with 3, usually less distinct white strigulae approximately opposite to distal 3 white strigulae from costa; basal dorsal strigula usually contiguous with second costal strigula. Apex of forewing with a large black apical spot, which is rarely reduced; fringe mostly light grey. Hindwing, including fringe, uniformly grey. Foreleg mostly dark fuscous dorsally, white ventrally, with 2 white annuli around basal tarsomeres; midleg with 2 oblique bands of white dorsally over tibia; tarsomeres more broadly banded with white dorsally; hindleg mostly white with much of tibia pale fuscous dorsally, and with 3 broad, pale fuscous annuli dorsally over tarsomeres.
Abdomen: dark fuscous dorsally and white ventrally with greyish suffusion on anterior portion of segments 2–7 laterally and sometimes ventrally on A8.
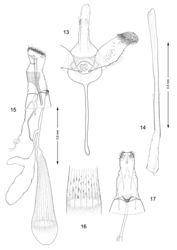
Male genitalia (Figs 13, 14): Valva relatively simple, similar to Macrosaccus morrisella in form, gradually constricted before apex; apex rounded, densely setose; base of costa fused to moderately thickened, arched transtilla; transtilla with rounded knoblike lateral projections that extend anteriorly in repose (more caudally when valvae are spread widely apart); saccus a slender, elongate rod ~ 1.2× length of valva. Aedeagus very long and uniformly slender, ~ 2.1× length of valva.
Female genitalia (Figs 15–17): Ductus bursae long and slender, nearly half the length of elongate corpus bursae. Accessory bursae ~ 2/3 the length of corpus bursae, arising from anterior 1/3 of ductus bursae; with a smaller lateral pouch arising ~ midway along side of accessory bursae. Corpus bursae gradually broadening anteriorly, with faint longitudinal striae in wall which bear longitudinal rows of low, dentate ridges around anterior third of corpus bursae; walls of anterior end (distal 1/5) of corpus bursae entirely membranous.
Larva
(Figs 59–80, 90–96). Hypermetamorphic; five larval instars. Earliest instars (1–3) highly modified sapfeeders with strongly depressed bodies and reduced chaetotaxy; maximum length 3.7 mm, width (T1): 0.9 mm. Later instars (4 and 5) tissue feeders, with cylindrical bodies; maximum length: 4.7 mm, width: 0.7 mm; body colour pale green to white with notal plates and pinnacula smooth, reduced and unpigmented (indistinct).
Sap-feeding instars
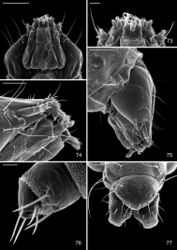
Head: Maximum width (third instar) 0.4 mm; greatly depressed, triangular. Most setae lost or reduced; 3 pairs of stemmata arranged in a lateral, anterior cluster on head. Labrum (Fig. 60) short and broad, bilobed, with 2 pairs of extremely reduced, peglike dorsal setae; anterior margin broadly concave, roughened, with 4–5 minute dentations along inner margin of lateral lobes. Mandibles broadly rounded, flattened, with 2 short cusps lateral to relatively large inner plate. Labium smooth, lateral margins subparallel; anterior margin shallowly notched at middle; spinneret absent. Maxillary and labial palpi absent. Hypopharynx broad, densely covered with minute spines along anterior margin; with margin slightly excavated at middle. Antenna 3-segmented, with short basiconic sensilla as shown (Fig. 62). Body: Setae generally reduced. Legs, prolegs, and crochets absent.
Tissue-feeding instars
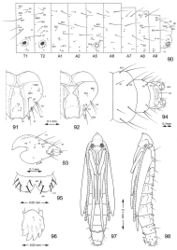
Head: Approximately round with full complement of mouthparts; brown; maximum width (fifth instar) 0.35 mm. Frons elongate, ~ 0.85× the distance to epicranial notch. Ecdysial line terminating near epicranial notch. Chaetotaxy (Figs 91–92) relatively complete; all three MD setae present, arising caudad to P1. P1 arising adjacent to ecdysial line. P2 reduced, arising slightly caudad to reduced L1. Setae AF1–2 absent. A2 arising near A3 in a line between P1 and A3. C1 and 2 reduced, closely adjacent. Four stemmata present. Antenna 3-segmented; first segment moderately long; sensilla as shown in Fig. 70. Labrum (Figs 68, 95) strongly bilobed with raised median portion on each lobe; M1 absent; numerous secondary spines visible from inner, ventral perimeter of labrum. Mandible (Figs 71, 96) with three large median cusps and one smaller median and two lateral cusps; mandibular setae variable (1–2) and located on anterior surface. Hypopharynx with dense, well developed dorsal spines. Maxilla as shown in Fig. 69. Spinneret a relatively short tube with a simple, rounded apex. Labial palpus with a relatively long basal segment bearing one short sensillum and a much shorter (~ 0.25× length of basal segment) apical bearing a single long apical sensillum ~ 2× length of apical segment. Thorax: Setae XD1 and 2 short, of equal lengths on prothorax (T1). SD1 elongate, immediately ventral to XD2; SD2 absent on T1, present on T2–3L group bisenose on T1–3. SV unisetose on T1–3. Legs (Fig. 76) relatively short but fully developed; coxae widely separated, with 4 coxal setae; pretarsal claw moderately curved. Abdomen: D and SD groups bisetose on A1–8, 10; unisetose on A9; L group bisetose on A1–5, unisetose on A6–10. Prolegs present on A3–5, 10; crochets of A3–5 consisting of 17–24 small hooks arranged in a uniordinal circle; anal proleg with crochets consisting of 15–18 small hooks arranged in a uniordinal semicircle opened caudally (Fig. 79). Anal plate with 4 pairs of setae.
Larval mine
(Figs 36–40). The mine begins as an elongate serpentine track (Fig. 37) which enlarges to an elongate-oval, whitish blotch Fig. 36, 38) located on one side of the midrib and usually on the under (abaxial) side of the leaflet. Eventually the mine becomes slightly tentiform due to the silk laid down by the later instar larvae.
Hosts
(Table 1). Fabaceae: Robinia pseudoacacia L. (Clemens 1859[1]: 320), Robinia viscosa Vent. (Chambers 1878[28]: 111), Robinia hispida L. (Chambers 1878[28]: 111; Needham et al. 1928[29]: 288). The primary host, Robinia pseudoacacia, is believed once to have occurred primarily in two regions within the United States – one centered in the Appalachian Mountains from central Pennsylvania to northern Georgia and Alabama, and the other in the Ozark Plateau of southern Missouri, eastern Arkansas to eastern Oklahoma. This tree has since spread over much of the continental United States, portions of northeastern Canada, and parts of South America, Europe, Asia, Africa, and Australia (Stone 2009[30]).
Life history
(Figs 36–40). The egg of Macrosaccus robiniella is deposited externally usually some distance from the leaf edge or midrib. Five larval instars have been observed by counting head capsules within mines in North America and Belgium. Kasch and Nicolai (2002)[31] reported up to six instars based on head capsule measurements in Germany. The larvae typically form elongate-oval, whitish blotch mines on usually the under (abaxial) side of the leaflets. Upon eclosion, the apodal, prognathous sap-feeding larva enters the leaf and begins a slender, subepidermal, serpentine mine (Fig. 37). Eventually the mine is expanded into an oval blotch (Fig. 38) which usually encompasses and obliterates the previous serpentine mine. As is true for the larvae of Phyllonorycter (Davis and Deschka 2001[32]), the last sap-feeding instar probably begins expanding the mine laterally. Initiation of the tissue-feeding instar is indicated by deeper feeding into the spongy and palisade tissue layers of the leaflet as the larva begins to ingest solid tissue. The resulting injury becomes visible from the opposite leaf surface, particularly in the underside mines, as dense, whitish punctures. As the tissue-feeding larva matures, it begins to lay down silken strands across the inner surface of the mine causing the leaflet to roll inwards and the mine to become tentiform (Fig. 39). Pupation occurs inside a silken cocoon (Fig. 40) within the mine without any precut exit opening. Especially during heavy infestations, the mines of adjacent larvae may coalesce resulting in multiple pupal cocoons. The phenology of this species has not been accurately determined over its range within North America. Normally two to three generations per year have been reported in Europe, which can occasionally reach as many as four (Nicolai 2005[33]).
Braun (1908)[12] noted that the mines could occur on both leaf surfaces. Weaver and Dorsey (1967)[19] described the larval mining behaviour of Macrosaccus robiniella in great detail and observed several differences between the upper side mines, which reportedly were more common at higher elevations (~ 760 m), and the under side mines. The latter were found most frequently at elevations of ~ 270 m at their West Virginia study sites. Some of the distinctions they observed were that upper side mines occurred usually more basal on the leaflet and often extended across the midrib, with the larval frass concentrated more basally within the mine. Under side mines are situated less basally and usually restricted to one side of the midrib, with frass scattered more uniformly throughout the mine. Only a single, somewhat loosely woven cocoon was observed in the upper side mines, compared to as many as three, densely woven cocoons in the lower mines. DRD compared males reared from the upper and lower side mines and found no significant morphological differences (Weaver and Dorsey 1967[19]). A search for the Weaver specimens in the collections of the University of West Virginia at Morgantown yielded no material associated with the upper side mines from the higher elevation sites (~ 760 m). Hopefully specimens from the higher elevation, upper side mines can be collected in order to examine their genetic distances.
In addition to Hymenoptera parasitoids, other Lepidoptera larvae have been noted within the mines of Macrosaccus robiniella (Weaver and Dorsey 1967[19]). These were observed to alter the appearance of the mine by removing all mesophyll and largely destroying the frass pattern created by Macrosaccus robiniella. Packard (1890)[34] identifies a species of Gelechiidae, Filatima pseudacaciella (Chambers), which sometimes feeds within the mine in addition to feeding externally.
Natural enemies
(Table 4). Fifty seven species (including two unidentified) ofHymenoptera, the great majority of which are members of Eulophidae (Noyes 2010[35]), have been reported as parasitoids of Macrosaccus robiniella in Europe and North America. Weaver and Dorsey (1967)[19] also list two species of predators in the families Reduviidae and Vespidae that preyed on Macrosaccus robiniella
Pupa
(Figs 82–89, 97, 98). Maximum length 3.6 mm; width 0.9 mm. Vertex with frontal process (cocoon cutter) relatively short, broadly triangular, acute (Figs 81, 82). Forewing extending to anterior margin of A6; antenna slightly longer to middle of A6; hindleg extending to A7. Abdomen mostly covered dorsally and ventrally with dense, minute spines; dorsum of A2–7 with a single anterior row of short, stout spines (Figs 83, 98); caudal half of sternum 7 with a transverse ridge (accessory cremaster) bearing ~ 18–21 mostly longitudinal rows of short, blunt spines (Figs 84, 85). Cremaster of A10 greatly reduced, nearly absent, consisting of 1–2 pairs of minute tergal spines.
| Parasitoid name | Family | Country | Reference |
|---|---|---|---|
| Achrysocharoides cilla (Walker, 1839) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Achrysocharoides gahani (Miller, 1962) | Eulophidae | Italy | Navone 2003[37]: 79 |
| Achrysocharoides gahani (Miller, 1962) | Eulophidae | Switzerland | Girardoz et al. 2007[38]: 606 |
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | Austria | Hansson and Shevtsova 2010[39]: 34 |
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | Germany | Hansson and Shevtsova 2010[39]: 34 |
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | Hungary | Hansson and Shevtsova 2010[39]: 34 |
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | Italy | Hansson and Shevtsova 2010[39]: 34 |
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | U.S.A. | Hansson and Shevtsova 2010[39]: 34 |
| Achrysocharoides robinicolus Hansson & Shevtsova, 2010 | Eulophidae | U.S.A. | Hansson and Shevtsova 2010[39]: 34 |
| Ageniaspis testaceipes (Ratzeburg, 1848) | Encyrtidae | Hungary | Csóka et al. 2009[36]: 407 |
| Apanteles nanus Reinhard, 1880 | Braconidae | Italy | Bolchi Serini 1990[40]: 142 |
| Astichus trifasciatipennis (Girault, 1913) | Eulophidae | Italy | Noyes 2010[35]: Internet |
| Baryscapus nigroviolaceus (Nees, 1834) | Eulophidae | Czech Republic | Girardoz et al. 2007[38]: 608 |
| Baryscapus nigroviolaceus (Nees, 1834) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Baryscapus nigroviolaceus (Nees, 1834) | Eulophidae | Italy | Gibogini et al. 1996[41]: 16 |
| Baryscapus nigroviolaceus (Nees, 1834) | Eulophidae | Switzerland | Girardoz et al. 2007[38]: 606 |
| Chrysocharis laomedon (Walker, 1839) | Eulophidae | Italy | Gibogini et al. 1996[41]: 16 |
| Chrysocharis nephereus (Walker, 1839) | Eulophidae | Switzerland | Whitebread 1990[42]: 349 |
| Chrysocharis pentheus (Walker, 1839) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Chrysocharis pentheus (Walker, 1839) | Eulophidae | Switzerland | Girardoz et al. 2007[38]: 606 |
| Cirrospilus elegantissimus Westwood, 1832 | Eulophidae | Italy | Gibogini et al. 1996[41]: 16 |
| Cirrospilus lyncus Walker, 1838 | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Cirrospilus talitzkii Bouček, 1961 | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Cirrospilus variegatus (Masi, 1907) | Eulophidae | Italy | Gibogini et al. 1996[41]: 16 |
| Cirrospilus viticola (Rondani, 1877) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Cirrospilus viticola (Rondani, 1877) | Eulophidae | Italy | Gibogini et al. 1996[41]: 16 |
| Closterocerus cinctipennis Ashmead, 1888 | Eulophidae | U.S.A. | Weaver and Dorsey 1965[18]: 934 |
| Closterocerus sp. | Eulophidae | Czech Republic | Girardoz et al. 2007[38]: 608 |
| Closterocerus trifasciatus Westwood, 1833 | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Closterocerus trifasciatus Westwood, 1833 | Eulophidae | Italy | Bolchi Serini 1990[40]: 143 |
| Colastes braconius Haliday, 1833 | Braconidae | Italy | Bolchi Serini 1990[40]: 142 |
| Colastes braconius Haliday, 1833 | Braconidae | Switzerland | Whitebread 1990[42]: 349 |
| Elachertus inunctus Nees, 1834 | Eulophidae | Italy | Zhu and Huang 2001[43]: 343 |
| Eupelmus urozonus Dalman, 1820 | Eupelmidae | Hungary | Csóka et al. 2009[36]: 407 |
| Hockeria unicolor Walker, 1834 | Chalcididae | Italy | Gibogini et al. 1996[41]: 16 |
| Horismenus fraternus (Fitch, 1856) | Eulophidae | U.S.A. | Weaver and Dorsey 1965[18]: 934 |
| Mesochorus sp. | Ichneumonidae | USA | Weaver and Dorsey 1967[19]: 180 |
| Minotetrastichus frontalis (Nees, 1834) | Eulophidae | Czech Republic | Girardoz et al. 2007[38]: 608 |
| Minotetrastichus frontalis (Nees, 1834) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Minotetrastichus frontalis (Nees, 1834) | Eulophidae | Italy | Bolchi Serini et al. 1990[40]: 143 |
| Minotetrastichus frontalis (Nees, 1834) | Eulophidae | Switzerland | Whitebread 1990[42]: 349 |
| Necremnus hungaricus (Erdös, 1951) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Neochrysocharis formosus (Westwood, 1833) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Pediobius bucculatricis (Gahan, 1927) | Eulophidae | Canada | Peck 1985[44]: 677 |
| Pediobius liocephalatus Peck, 1985 | Eulophidae | Canada | Peck 1985[44]: 675 |
| Pediobius saulius (Walker, 1839) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Pediobius saulius (Walker, 1839) | Eulophidae | Italy | Gibogini et al. 1996[41]: 16 |
| Pholetesor circumscriptus Nees, 1834 | Braconidae | Italy | Bolchi Serini 1990[40]: 142 |
| Pholetesor nanus (Reinhard, 1880) | Braconidae | Czech Republic | Girardoz et al. 2007[38]: 608 |
| Pholetesor nanus (Reinhard, 1880) | Braconidae | Hungary | Csóka et al. 2009[36]: 407 |
| Pholetesor nanus (Reinhard, 1880) | Braconidae | Italy | Bolchi Serini 1990[40]: 142 |
| Pholetesor nanus (Reinhard, 1880) | Braconidae | Switzerland | Girardoz et al. 2007[38]: 606 |
| Pholetesor ornigis Weed, 1887 | Braconidae | U.S.A. | Weaver and Dorsey 1965[18]: 934 |
| Pnigalio agraules (Walker, 1839) | Eulophidae | Switzerland | Girardoz et al. 2007[38]: 606 |
| Pnigalio pectinicornis (Linnaeus, 1758) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Pnigalio pectinicornis (Linnaeus, 1758) | Eulophidae | Italy | Bolchi Serini 1990[40]: 143 |
| Pnigalio pectinicornis (Linnaeus, 1758) | Eulophidae | Switzerland | Girardoz et al. 2007[38]: 606 |
| Pnigalio soemius (Walker, 1839) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Pnigalio soemius (Walker, 1839) | Eulophidae | Italy | Bolchi Serini 1990[40]: 143 |
| Pteromalus chrysos Walker, 1836 | Pteromalidae | Italy | Gibogini et al. 1996[41]: 16 |
| Pteromalus sp. | Pteromalidae | Czech Republic | Girardoz et al. 2007[38]: 608 |
| Sympiesis acalle (Walker, 1848) | Eulophidae | Hungary | Szabóky and Csóka 1997[45]: 570 |
| Sympiesis acalle (Walker, 1848) | Eulophidae | Italy | Bolchi Serini 1990[40]: 143 |
| Sympiesis dolichogaster Ashmead, 1888 | Eulophidae | Switzerland | Girardoz et al. 2007[38]: 607 |
| Sympiesis gordius (Walker, 1839) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Sympiesis gordius (Walker, 1839) | Eulophidae | U.S.A | Weaver and Dorsey 1965[18]: 934 |
| Sympiesis marylandensis Girault, 1917 | Eulophidae | U.S.A. | Maier 1988[46]: 731 |
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | Czech Republic | Girardoz et al. 2007[38]: 608 |
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | Hungary | Csóka et al. 2009[36]: 407 |
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | Italy | Bolchi Serini 1990[40]: 143 |
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | Switzerland | Girardoz et al. 2007[38]: 607 |
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | U.S.A. | Weaver and Dorsey 1965[18]: 934 |
Types
Lithocolletis robiniella Clemens: Lectotype ♀ (present designation): “14”; “Lithocolletis robiniella Clemens, Type ! A.B. 1902; Type 7505 Lithocolletis robiniella B. Clemens”; “Lectotype ♀ by D. R. Davis”, (ANSP). [The abdomen, right forewing, and distal part of right hindwing are missing].
Paralectotypes 3 ♂ and 1 specimen without abdomen “Syntype”, “Lithocolletis robiniella Clem. 1/4”, “Clemens det. ex Clemens coll.”, “Stainton coll. Brit. Mus. 1893–134”; same labels except nrs. 2/4, 3/4 and 4/4. The specimen with nr. 4/4 carries an extra label in Stainton’s handwriting: “Lithocolletis robiniella Clemens, Proc. N. S. Phil. 1859 p. 319, n.s. unlike any European species”, (BMNH).
Argyromiges pseudacaciella Fitch: Lectotype ♀ (present designation): “Argyromiges Pseudacaciella; Type No. 514 U.S.N.M”; “Lectotype ♀ by D. R. Davis.” (USNM).
Material examined
BELGIUM: Province of Antwerp: Postel: 15 ♂, 23 ♀, 7 Sep 2009, em. 15–22 Sep 2009, J. and W. De Prins, leafmine on Robinia pseudoacacia, USNM slides 34257, 34258, 34263, DNA/BOLD ID RDOPO090-09, GenBank GU669590, DNA/BOLD ID RDOPO091-09, GenBank GU669591, (USNM). CANADA: ONTARIO: Ancaster: 1 ♂, 24 Jul 1964, T. N. Freeman, Host: Robinia pseudoacacia, 64–20, (CNC). Bobcaygeon: 1 ♂, 23 Jul 1932, J. McDunnough, reared on Robinia, (CNC). Ottawa: 1 ♂, 26 Aug 1955, G. G. Lewis, Host: B. locust, 55–8, (CNC). Walsh: 1 ♂, 23 Sep 1966, T. N. Freeman, Host: Bl. Locust, (CNC). UNITED STATES: DISTRICT OF COLUMBIA: 1 ♀, 7 Jul 1879, Host: Robinia, V.T. Chambers, (USNM); 3 ♂, 2 ♀, 9 Aug 1898, (USNM); 1 ♀, 24 Aug 1899 (USNM); 4 UNK, 18 Sep 1899, Host: Robinia, (USNM); Rock Creek Park: 1 ♂, 22 May 1984, W. E. Steiner, (USNM). ILLINOIS: Adams Co: Quincy: 3 ♂, 3 ♀, 15–21 Feb 1948; 2 ♂, 2 ♀, 6 Apr 1947; J. P. Nielson (INHS). Coles Co: Fox Ridge State Park: 1 ♂, 1 Jun 1991, em. 9 Jun, 1991; 1 ♀, 29 Jun 1991, em. 5 Jul 1991; 3 ♂, 1 ♀, 14 Jul 1991, em. 16–20 Jul 1991, T. Harrison, leafmine on Robinia pseudoacacia, (INHS); 1 ♂, 7 Jun 1991, at UV light T. Harrison, (INHS). Putnam Co: 1 ♀, 15 Apr 1996; 1 ♂, 24 Apr 1963; 1 ♂, 29 Apr 1969; 1 ♂, 2 Jun 1966; 1 ♀, 11 Jul 1959; 1 ♀, 3 Sep 1951, M. O. Glenn, (INHS); 1 ♀, 3 May 1953; 1 ♂, 1 ♀, 10 May 1953; 1 ♂, 26 Aug 1961; 2 ♂, 10 Sep 1948; 2 ♂, 10 Oct 1948, M. O. Glenn, reared from Robinia pseudoacacia, (INHS). Vermilion Co: Kickapoo State Recreation Area: 1 ♂, 13 Jun 1991, em. 14 Jun 1991, T. Harrison, leafmine on Robinia pseudoacacia, (INHS). KENTUCKY: Fayette Co: Lexington: 1 ♂, 6–13 Oct 1975, malaise trap, (USNM). MARYLAND: Garret Co: Deep Creek. Lake State Park: 15 ♂, 12 ♀, 16 Sep 1990, em. 23 Sep – 7 Oct 1990, D. and S. Davis, DRD 821, Host: Robinia pseudoacacia L. USNM slides 33282, 30903, 30895, 30894, DNA/BOLD ID RDOPO088-09, GenBank GU669592, DNA/BOLD ID RDOPO089-09, GenBank GU669593, (USNM). Montgomery Co: Fort Washington, vicinity Henson Creek: 1 ♂, 19 Sep 1990, em. 13 Oct 1993, D. Davis, DRD 1376, Host: Robinia pseudoacacia L., (USNM). MASSACHUSETTS: Essex Co: Beverly: 1 ♂, 2/69, Burgess, (BMNH). MICHIGAN: Clinton Co: T6N-R1W S10: 3 ♂, 2 Oct 1997, em. 9–13 Oct 1997, R. J. Priest, Host: Robinia pseudoacacia L., (USNM). Wayne Co: Detroit: 1 ♂, 2 ♀, 20 Nov 1995, T. Wallenmeier, (USNM). MISSOURI: Boone Co: Columbia: 1 ♂, 25 Nov 1995, slide USNM 17047, 1 ♀, 14 Dec 1969, W.S. Craig, under bark of sycamore, (USNM). NEW HAMPSHIRE: Cumberland Co: Hampton: 1 ♀, 16 Feb 1906, S.A. Shaw, (USNM). NEW JERSEY: Burlingnton Co: Moorestown: 2 ♂, 22 Aug 1902, W.D. Kearfott, Host: Locust, (USNM). NEW YORK: Specific locality unknown: 1 ♀, lectotype, Argyromiges pseudacaciella Fitch, (USNM). Clinton Co: Peru: 2 ♂, 2–18 May 1977, R. Weires, caught in pheromone trap, slide USNM 20912, (USNM). Essex Co: Crown Point: 2 ♂, 4–20 May 1977, 1 ♂, 20 May-17 Jun 1977, R. Weires, caught in pheromone trap, slide USNM 20910, (USNM). Livingston Co: Letchworth State Park: 12 ♂, 8 ♀, 21–22 Jun 1986, E. R. Hoebeke, reared from mines of Robinia pseudoacacia, (CU). Thompkins Co: Ithaca: 1 ♂, 15 Feb; 1 ♂, 1 ♀, 8 Apr 1945, Renwick, (CU). NORTH CAROLINA: Macon Co: Highlands, 3865’: 6 ♂, 5 ♀, 1–24 Aug 1958, R. W. Hodges, (CU); 11 ♂, 5 ♀, 27 Jul-25 Aug 1958, R.W. Hodges, (USNM). OHIO: Hamilton Co: Cincinnati: 1 ♂, 29 Apr 1905, 6806, (CNC); 1 ♀, 29 Apr 1903, 7 ♂, 4 May 1904, 1 ♂, 23 July 1903, 1 ♂, 1 ♀, 25–27 Sep 1902, 1 ♂, 1 ♀, 30 Sep 1911, slide USNM 97837, 2 ♀, 10–22 Oct 1903, 3 ♂, 15–20 Nov 1903, Annette F. Braun, (USNM). PENNSYLVANIA: Specific locality unknown: 1 ♀, lectotype, Lithocolletis robiniella Clemens, (ANSP). Allegheny Co: Oak Station: 1 ♂, 1 ♀, 1 Oct 1910, Fred Marloff, (CU); 1 ♀, 10 Apr 1910, 3 ♂, 5 ♀, 3–22 May 1910, 1 ♀, 12 Jun 1908, Fred Marloff, (USNM). Erie Co: Girard: 3 ♂, 1 ♀, 9 Oct 1920, reared from black locust, (CU). Franklin Co: Mont Alto: 1 ♀, 5 Oct 1971, reared Black locust seedling, slide USNM 17166, (USNM). Indiana Co: Strongstown: 1 ♀, 23 Sep 1971, reared Black locust seedling, (USNM). Monroe Co: Sciota: 1 ♂, 21 Jul 1965, T. N. Freeman, Host: Robinia pseudoacacia, 65–27, (CNC). SOUTH CAROLINA: Oconee Co: Cherry Hill Rec. Area, Rt.107, 2000’ [610m]: 2 ♂, 1 ♀, 11 Aug 1958, R.W. Hodges, (CU); 3 ♂, 11 Aug 1958, R.W. Hodges, slide USNM 17017, (USNM). TEXAS: 1 ♀, Boll, (USNM). VIRGINIA: Arlington Co: Rosslyn: 1 ♂, A. Busck, underside mine on Hog peanut, (USNM). Madison Co: Shenandoah Nat. Park, Skyline: 3 ♀, 12 Aug 1972, E. Jäckh, Host: Robinia pseudoacacia L., (USNM). WEST VIRGINIA: Marion Co: Morgantown: 5 ♂, 3 ♀, [no date], Host: Robinia, slide USNM 97835, (USNM). WISCONSIN: Dane Co: Madison: 1 ♂, 1 ♀, 24 Aug 1958, L. J. Bayer, Host: Robinia, (USNM).
Distribution
Macrosaccus robiniella occurs naturally over much of eastern North America from Ontario, Canada south to South Carolina and west to Missouri and Texas. Macrosaccus robiniella was first reported in Europe in 1983, near Basel, Switzerland (Whitebread 1990[42]: 344) and has since spread through 23 European countries (Table 5).
| Country | First year of occurrence | Reference to the first record |
|---|---|---|
| Albania | not recorded | Lopez-Vaamonde et al. 2010[47]: 645 |
| Austria | 1991 | Huemer et al. 1992[48]: 199 |
| Belgium | 2001 | De Prins and Groenen 2001[25]: 159 |
| Bosnia and Herzegovina | 1999 | Dimić et al. 2000[49]: 7 |
| Bulgaria | 2001 | Tomov 2003[50]: 105 |
| Croatia | 2000, unpublished observations, Aleš Laštůvka & Hana Šefrová, pers. comm. | Lopez-Vaamonde et al. 2010[47]: 645 |
| Czech Republic | 1992 | Laštůvka et al. 1993[51]: 31 |
| Denmark | 2003 | Buhl et al. 2005[52]: 79 |
| France | 1984 | Whitebread and Joos 1986[53]: 117 |
| Germany | 1988–1989 | Whitebread 1990[42]: 345 |
| Hungary | 1992 | Szabóky and Csóka 1997[45]: 569 |
| Italy | 1988 | Bolchi Serini and Trematerra 1989[54]: 193 |
| Lithuania | 2007 | Noreika 2008[55]: 35 |
| Moldova(Pridnestrovje) | 2006 | Antyukhova 2007[56]: 65 |
| Netherlands | 1999 | De Prins and Groenen 2001[25]: 160 |
| Poland | 1999 from Šefrová 2002[57]: 10 | Buszko and Novacki 2000[58]: 25 |
| Romania | 2002 | Neţoiu 2003[59]: 154 |
| Serbia | 1998 | Dimić et al. 1999[60]: 34 |
| Slovakia | 1992 | Buszko 1996[61]: 53 |
| Slovenia | 1994 | Seljak 1995[62]: 78 |
| Spain | 2001 | Olivella 2002[63]: 35 |
| Switzerland | 1983 | Whitebread and Joos 1986[53]: 117 |
| Ukraine | 2002 | Bidzilya and Budashkin 2004[64]: 61 |
Remarks
Thesynonymousnames Lithocolletis robiniella Clemens and Argyromiges pseudacaciella Fitch were both published in 1859. The month of publication for robiniella is clearly indicated as November in the Proceedings of the Academy of Natural Sciences of Philadelphia for that year. The month of publication for pseudacaciella Fitch cannot be determined as precisely. With the assistance of Tim McCabe of the New York State Museum, we were able to resolve an approximate date of printing for the Fifth report of Fitch’s Report on the noxious, beneficial and other insects of the state of New York (Fitch 1859[26]), but we were not able to determine the actual distribution date. From such dated sections of that Report, particularly a “Notice “ to the farmers of New York, McCabe deduced that the Fifth Report most likely was printed in March, 1859. Attempts to locate receivership stamps for this report in various libraries to determine an approximate distribution date have been unsuccessful.
Thus, available evidence now suggests that pseudacaciella Fitch preceded the publication of robiniella Clemens by a few months. Because it is known that (1) Riley (1891)[9] first treated pseudacaciella as a junior synonym of robiniella and no subsequent author has considered it otherwise, and (2) that the name robiniella has been recognized as the valid name for this taxon in more than 25 publications (141 publications using robiniella as the valid name are actually known) by more than 10 authors, this name must be preserved as the valid name for this taxon in accordance with the provisions of article 23.9.1 of the International Code of Zoological Nomenclature (ICZN 1999[65]).
Neither the type locality nor the number of specimens examined were provided by Fitch for Argyromiges pseudacaciella. The same is true for the other two species of Gracillariidae Fitch proposed in 1859, Argyromiges morrisella, and Argyromiges uhlerella. Because it is believed that most of Fitch’s collecting occurred within the vicinity of his “bug house” (still standing and now a historical site) in Salem, New York, it is likely that the type locality for all three species may be from this general area (McCabe, in litt.).
Taxon Treatment
- Davis, D; De Prins, J; 2011: Systematics and biology of the new genus Macrosaccus with descriptions of two new species (Lepidoptera, Gracillariidae) ZooKeys, 98: 29-82. doi
Other References
- ↑ 1.0 1.1 Clemens B (1859) Contribution to American Lepidopterology. No. 2, Proceedings of the Academy of Natural Sciences of Philadelphia [1859]: 317–328.
- ↑ Clemens B (1872) Micro-Lepidopterous Larvae. In: Stainton H (Ed) The Tineina of North America. John van Voorst, London, 161–178.
- ↑ Chambers V (1871) Micro-Lepidoptera. Canadian Entomologist 3: 54–58, 84–88, 108–112, 127–130, 146–149, 161–166, 182–185, 205–209, 221–224. doi:10.4039/Ent354-3
- ↑ Chambers V (1872) Micro-Lepidoptera. Canadian Entomologist 4: 7–12, 25–29, 41–44, 65–69, 88–92, 106–108. doi:10.4039/Ent47-1
- ↑ Chambers V (1875) Tineina of the United States. Cincinnati Quarterly Journal of Science 2:226-259.
- ↑ Chambers V (1877) Art. VI. The Tineina of Colorado. Bulletin of the United States Geological Survey 3:121-145.
- ↑ Zeller P (1875) Beiträge zur Kenntniss der nordamericanischen Nachtfalter, besonders der Microlepidopteren. Dritte Abheilung. Verhandlungen der zoologisch-botanischen Gesellshaft in Wien 25: 205–360, pls. 8–10.
- ↑ Frey H, Boll J (1878) Tineen aus Texas. Entomologische Zeitung, Stettin, 39:249-279.
- ↑ 9.0 9.1 Riley C (1891) Tineina. In: Smith J. List of the Lepidoptera of Boreal America. American Entomological Society, Philadelphia, 94–114.
- ↑ Busck A (1903) Notes on Brackenridge Clemens’ types of Tineina. Proceedings of the Entomological Society of Washington 5:181-220.
- ↑ 11.0 11.1 Dyar H (1903) A list of North American Lepidoptera. United States National Museum Bulletin 52 (1902): xix + 723.
- ↑ 12.0 12.1 12.2 Braun A (1908) Revision of the North American species of the genus Lithocolletis Hübner. Transactions of the American Entomological Society 34: 269–357, pls. xx-xxiv.
- ↑ 13.0 13.1 Meyrick E (1912a) Lepidoptera Heterocera (Tineae). Fam. Gracilariadae [sic]. In: Wytsman P (Ed) Genera Insectorum. Fascicule 128. V. Verteneuil & L. Desmet, Imprimeurs-Éditeurs, 1–36.
- ↑ 14.0 14.1 Meyrick E (1912b) Adelidae, Micropterigidae, Gracilariadae [sic]. In: Wagner H (Ed) Lepidopterorum Catalogus, Pars 6, W. Junk, Berlin, 25–68.
- ↑ Braun A (1914) Evolution of the color pattern in the microlepidopterous genus Lithocolletis. Journal of the Academy of Natural Sciences, Philadelphia 16: 103–168, pls. iii–iv.
- ↑ Forbes W (1923) The Lepidoptera of New York and neighboring states, Part 1, Primitive forms, Microlepidoptera, Pyraloids, Bombyces. Cornell University Agriculture Experiment Station. Memoir 68:1-729.
- ↑ 17.0 17.1 McDunnough J (1939) Check List of the Lepidoptera of Canada and the United States of America. Part II. Microlepidoptera. Memoirs of the Southern California Academy of Sciences 2:1-171.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 Weaver J, Dorsey C (1965) Parasites and predators associated with five species of leaf-mining insects in black locust. Annals of the Entomological Society of America 58:933-934.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 Weaver J, Dorsey C (1967) Larval mine characteristics of five species of leaf-mining insects in black locust, Robinia pseudoacacia. Annals of the Entomological Society of America 60:172-186.
- ↑ 20.0 20.1 Ely C (1918) A revision of the North American Gracilariidae [sic] from the standpoint of venation. Proceedings of the Entomological Society of Washington 19:29-77.
- ↑ 21.0 21.1 Davis D (1983) Gracillariidae. In: Hodges R Dominick T Davis D Ferguson D Franclemont J Munroe E Powell J (Eds) Check List of the Lepidoptera of North America North of Mexico. E.W. Classey Ltd. and the Wedge Entomological Research Foundation, London, 9–11.
- ↑ Maier C, Davis D (1989) Southern New England host and distributional records of Lithocolletine Gracillariidae (Lepidoptera) with comparison of host specificity in temperate regions. Entomological Society of America, Miscellaneous Publications, No. 70: 1–23.
- ↑ 23.0 23.1 Leraut P (1997) Liste systématique et synonymique des lépidoptères de France, Belgique et Corse (deuxième edition). Alexanor Supplement: 1–526.
- ↑ 24.0 24.1 De Prins W, De Prins J (2005) Gracillariidae (Lepidoptera). In: Landry B (Ed) World Catalogue of Insects, vol. 6. Apollo Books, Stenstrup, 1–502.
- ↑ 25.0 25.1 25.2 25.3 De Prins J, De Prins W (2011) Global Taxonomic Database of Gracillariidae (Lepidoptera). http://www.gracillariidae.net [accessed 11 March 2011]
- ↑ 26.0 26.1 Fitch A (1859) Report on the noxious, beneficial and other insects of the state of New York. Fifth report. Transactions of the New York State Agricultural Society 18 (1858): 781-854, 9 Figs Also published separately with different pagination (p. 1–74) and with an index to parts 3–5, New York.
- ↑ Barnes W, McDunnough J (1917) Check List of the Lepidoptera of Boreal America. Herald Press, Decatur, Illinois, 392 pp.
- ↑ 28.0 28.1 Chambers V (1878) Art. IV. Tineina and their foodplants. Bulletin of the United States Geological and Geographical Survey of the Territories 4:107-124.
- ↑ Needham J, Frost S, Tothill B (1928) Leaf-mining Insects. The Williams and Wilkins Co., Baltimore, viii+351 pp.
- ↑ Stone K (2009) Robinia pseudoacacia. In: Fire effects information system. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Services Laboratory. http://www.fs.fed.us/database/feis/
- ↑ Kasch K, Nicolai V (2002) Phyllonorycter robiniella (Clemens) (Lepidoptera, Gracillariidae) – ein nordamerikanischer Schmetterling neu in Berlin. In: Kowarik I Starfinger U (. ): Biologische Invasionen. Herausforderung zum Handeln? Neobiota 1 :193-202..
- ↑ Davis D, Deschka G (2001) Biology and systematics of the North American Phyllonorycter leafminers on Salicaceae, with a synoptic catalogue of the Palearctic species (Lepidoptera: Gracillariidae). Smithsonian Contribution to Zoology 614: 1–89, Figs 1–451, 6 maps, 5 tables.
- ↑ Nicolai V (2005) The invasive leaf miner Phyllonorycter robiniella feeding on black locust, Robinia pseudoacacia, in Central Europe (Lepidoptera: Gracillariidae). Entomologia Generalis 28:193-200.
- ↑ Packard A (1890) Insects injurious to forest and shade trees. Fifth Report of the U.S. Entomological Commission. House of Representatives Miscellaneous Document 269:361-373.
- ↑ 35.0 35.1 Noyes J (2010) Universal Chalcidoidea Database, The Natural History Museum, London. http://www.nhm.ac.uk/research-curation/research/projects/chalcidoids [accessed 27 December 2010]
- ↑ 36.00 36.01 36.02 36.03 36.04 36.05 36.06 36.07 36.08 36.09 36.10 36.11 36.12 36.13 36.14 36.15 36.16 36.17 Csóka G, Pénzes Z, Hirka A, Mikó I, Matosevic D, George D (2009) Parasitoid assemblages of two invading block locust leaf miners, Phyllonorycter robiniella and Parectopa robiniella in Hungary. Periodicum biologorum 111:405-411.
- ↑ Navone P (2003) Reperimento in Italia di una specie neartica del genere Achrysocharoides (Hymenoptera Eulophidae), parassitoide di Phyllonorycter robiniella (Clemens). Bollettino di Zoologia agraria e Bachicoltura 35:79-82.
- ↑ 38.00 38.01 38.02 38.03 38.04 38.05 38.06 38.07 38.08 38.09 38.10 38.11 38.12 38.13 Girardoz S, Volter L, Tomov R, Quicke D, Kenis M (2007) Variations in parasitism in sympatric populations of three invasive leaf miners. Journal of Applied Entomology 131:603-612. doi:10.1111/j.1439-0418.2007.01146.x
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 Hansson C, Shevtsova E (2010) Three new species of Achrysocharoides Girault (Hymenoptera: Eulophidae) parasitoids of Phyllonorycter spp. (Lepidoptera: Gracillariidae) on Acer platanoides and Robinia pseudoacacia. Zootaxa 2388:23-43.
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 40.7 40.8 40.9 Bolchi Serini G (1990) Parassitoidi di Parectopa robiniella Clemens e di Phyllonorycter robiniellus (Clemens) (Lepidoptera Gracillariidae). Bollettino di Zoologia agrariae Bachicoltura 22:139-149.
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 41.6 41.7 Gibogini B, Alma A, Arzone A (1996) Bollettino di Zoologia agrariae Bachicoltura 28:13-22.
- ↑ 42.0 42.1 42.2 42.3 42.4 Whitebread S (1990) Phyllonorycter robiniella (Clemens, 1859) in Europe (Lepidoptera, Gracillariidae). Nota lepidopterologica 12 (1989):344-353.
- ↑ Zhu C, Huang D (2001) A Study of Chinese Elachertus spinola (Hymenoptera: Eulophidae). Zoological Studies 40:317-354.
- ↑ 44.0 44.1 Peck O (1985) The taxonomy of the Nearctic species of Pediobius (Hymenoptera: Eulophidae), especially Canadian and Alaskan forms. Canadian Entomologist 117: 647–704. doi:10.4039/Ent117647-6
- ↑ 45.0 45.1 Szabóky C, Csóka G (1997) A Phyllonorycter robiniella Clemens 1859 akáclevél aknázómoly megtelepedése Magyarországon. Növényvédelem 33:569-571.
- ↑ Maier C (1988) Gracillariid hosts of Sympiesis marylandensis (Hymenoptera: Eulophidae) in New England. Annals of the Entomological Society of America 81:728-732.
- ↑ 47.0 47.1 Lopez-Vaamonde C, Agassiz D, Augustin S, De Prins J, De Prins W, Gomboc S, Ivinskis P, Karsholt O, Koutroumpas A, Koutroumpa F, Laštůvka Z, Marabuto E, Olivella E, Przybyłowicz L, Roques A, Ryrholm N, Šefrová H, Šima P, Sims I, Sinev S, Skulev B, Tomov R, Zilli A, Lees D (2010) Lepidoptera. In: Roques A, Kenis M, Lees D, Lopez-Vaamonde C, Rabitsch W, Rasplus J-Y, Roy DB (Eds) Alien terrestrial arthropods of Europe. BioRisk 4:603-668.
- ↑ Huemer P, Deutsch H, Habeler H, Lichtenberger F (1992) Neue und bemerkenswerte Funde von Kleinschmetterlingen in Österreich. Bericht des naturwissenschaftlich-medizinischen Vereins in Innsbruck 79:199-202.
- ↑ Dimić N, Dautbasić M, Magud B (2000) Phyllonorycter robiniella Clemens, nowa vrsta minera lista u entomofauni Bosne i Herzegovine. Works of the Faculty of Forestry of the University of Sarajevo 1:7-15.
- ↑ Tomov R (2003) Phyllonorycter robiniella (Clemens, 1859) (Lepidoptera, Gracillariidae), a new pest on black locust Robinia pseudoacacia L. in Bulgaria. Proceedings, scientific papers. The 50th anniversary of University of Forestry Sofia, 105–107.
- ↑ Laštůvka Z (1993) Katalog von Faltern der Mährisch-Schlesischen Region (Lepidoptera). Landwirtschaftliche und Orstliche Universität, Brno, 130 pp.
- ↑ Buhl P, Falck P, Jorgensen B, Karsholt O, Larsen K, Vilhelmsen F (2005) Records of Microlepidoptera from Denmark in 2004 (Lepidoptera). Entomologiske Meddelelser 73:73-86.
- ↑ 53.0 53.1 Whitebread S, Joos R (1986) Nachtfalter und Kleinschmetterlinge. In: Blattner M Ritter M Ewald K (Eds) Basler Natur-Atlas I. Basler Naturschutz, Basle, 116–121.
- ↑ Bolchi Serini G, Trematerra P (1989) Comparsa del neartico Phyllonorycter robiniellus (Clemens) (Lepidoptera Gracillariidae) in Italia. Bollettino di Zoologia agrariae Bachicoltura 21:193-198.
- ↑ Noreika R (2008) Phyllonorycter robiniella (Clemens, 1859) (Lepidoptera: Gracillariidae) – a new species for the Lithuanian fauna. Naujos ir retos Lietuvos vabzdžių rūšys 19 (2007):35-38.
- ↑ Antyukhova O (2007) A review of mining Lepidoptera in the parks of the Pridnevstrovje region. [Антюхова, О. В. Обзор минеров парковой зоны в Приднестровском регионе]. Вестник Приднестровского университета 2:62-67.
- ↑ Šefrová H (2002) Phyllonorycter robiniella (Clemens, 1859) – egg, larva, bionomics and its spread in Europe (Lepidoptera, Gracillariidae). Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 50:7-12.
- ↑ Buszko J, Nowacki J (2000) The Lepidoptera of Poland. A Distributional Checklist. Polish Entomological Monographs Vol. 1. Polskie towarzystwo entomologiczne, Poznan, Toruń, 178 pp.
- ↑ Neţoiu C (2003) O nouă molie minieră la salcâmul din România- Phyllonorycter robiniella Clemens 1859 (Lepidoptera: Gracillariidae). Muzeul Olteniei Craiova, Studii şi comunicări, Ştiinţele Naturii 19:154-156.
- ↑ Dimić N, Graora D, Magud B, Perić P (1999) Opet jedna nova vrsta minera lista u entomofauni Jugoslavije. Biljni lecar, Novi Sad 27:34-37.
- ↑ Buszko J (1996) Gracillariidae. In: Karsholt O Razowski J (Eds) The Lepidoptera of Europe. A Distributional Checklist. Apollo Books, Stenstrup, 48–54.
- ↑ Seljak G (1995) Phyllonorycter robiniella (Clemens), še en nov listni zavrtač robinije v Sloveniji. Gozdarski vestnik 53:78-82.
- ↑ Olivella E (2002) Phyllonorycter robiniella (Clemens, 1859) arriba a la península Ibèrica (Lepidoptera: Gracillariidae). Butlletí de la Societat catalana de Lepidopterologia 87 (2001):35-38.
- ↑ Bidzilya O, Budashkin Y (2004) New records of Lepidoptera from Ukraine. Proceedings of Zoological Museum of Kiev Taras Shevchenko National University 2:59-68.
- ↑ ICZN International Commission on Zoological Nomenclature (1999) International code of zoological nomenclature. Fourth Edition. London: The International Trust for Zoological Nomenclature., c/o The Natural History Museum, London, UK, xxix+306 pp.
Images
|