Tipula recondita
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Pilipenko2012ZooKeys192, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Pilipenko2012ZooKeys192">{{Citation See also the citation download page at the journal. |
Ordo: Diptera
Familia: Tipulidae
Genus: Tipula
Name
Tipula recondita Pilipenko & Salmela sp. n. – Wikispecies link – ZooBank link – Pensoft Profile
Material examined
Holotype: Male, in alcohol (NCBN). “Finland, Lkoc: Kittilä, Iso Mustavaara, old-growth herb-rich forest, 67.6340°N, 25.4160°E, 30.V.–1.VII. 2009, J. Salmela leg.” (white label, printed) “Tipula (Pterelachisus) recondita sp. n./ Pilipenko & Salmela 2011[1]/ HOLOTYPE” (white label, printed) “BOLD sample ID JES-20110034” (white label, printed). Both wings are detached. Only one leg is present, other legs are missing. Tip of abdomen is detached and, including separate sperm pump, preserved in a microvial. This microvial is in the same tube as are wings and rest of the specimen. DNA barcode (524 bp) of holotype (coded JES-20110034|FINTI034-11|Tipula recondita):
ATGCTTTTATTATAATTTTTTTTATAGTTATACCTATTATAATTGGAGGATTTGGAAATTGATTAGTACCTTTAATATTAGGTGCCCCTGATATAGCCTTTCCTCGAATAAATAATATAAGTTTTTGAATATTACCTCCTTCACTTACTCTTTTATTAGCTAGTAGTATAGTCGAAAACGGTGCGGGGACTGGATGAACCGTTTATCCCCCACTCTCATCTAGAATTGCCCATACAGGAGCTTCAGTTGATTTAGCCATTTTTTCTCTTCATTTAGCTGGAATTTCTTCAATTTTAGGAGCAGTAAATTTTATTACTACAGTAATTAATATACGATCAAGAGGAATTACTTTAGACCGAATACCTTTATTTGTTTGATCGGTAGTAATTACTGCAGTATTATTACTACTCTCTTTACCTGTATTAGCGGGAGCTATTACTATACTTTTAACTGATCGAAATTTAAATACATCATTTTTTGATCCTGCAGGAGGTGGAGATCCAATTCTTTACCAACATTTATTT
Paratypes
Finland, Lkoc: Kittilä, Iso Mustavaara Nature reserve, herb-rich old-growth forest, 67.6340°N, 25.4160°E, 30.V.–1.VII. 2009, Malaise trap, J. Salmela leg., 2 males (ZMUT, in alcohol [BOLD sample ID JES-20110035] and a pinned specimen). DNA barcode (524 bp) of paratype (JES-20110035|FINTI035-11) is identical to the holotype sequence. Russia, Far East, Primorski kray, Kedrovaya Pad’, oak forest (Quercus mongolica), 43.1301°N, 131.5041°E, 7.VII. 2006 V. Pilipenko leg., 3 males and 3 females, deposited in ZSIP (BOLD sample ID JES-20110036), ZMUM, VPM. DNA barcode (524 bp) of paratype male (JES-20110036|FINTI036-11) differs from holotype at three positions (212=C, 473=T, and 515=G). In other words, intraspecific K2P distance between Finnish and Russian specimens was 0.2 %.
Diagnosis
Rather small yellowish brown Tipula species (body length: 11 mm male, 12.3 mm female; wing length 11–12.6 mm male, 12.5–13.5 mm female). Scape, pedicel and base of 1st flagellomere yellowish, other flagellomeres brown. Caudal margin of male 9th tergite with a median notch, bearing no tooth or other elevated structures. Outer gonostylus narrow, about as long as inner gonostylus, slightly bent sub-basally. Lower beak of inner gonostylus apically rounded, black. Outer basal lobe of inner gonostylus with 3–4 stout black spines.
Description
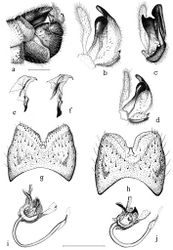
Male. Head gray pruinose, sparsely covered with dark hairs. Base of rostrum gray pruinose, otherwise dark brown, shining. Nasus distinct, tip with light bristles (Fig. 2a). Palpi brownish. Lengths of palpal segments (n=2): p1 128-147, p2 307-309, p3 317-365, p4 309-333 and p5 1207-1359. Scape, pedicel and base of 1st flagellomere yellowish, other flagellomeres brown. Scape cylindrical (length 442–466, width 119–120, n=2). Pedicel globular (length 132–134, width 134–135, n=2). Flagellar segments cylindrical, covered with silvery, erect and thick pubescence. Verticils black, shorter than respective segments (Fig. 2a). Lengths of flagellomeres (n=2): f1 371–398, f2 312–314, f3 298–316, f4 289–326, f5 297–324, f6 296–325, f7 291, f8 270–289, f9 257–261, f10 227–230 and f11 100. Thorax. General coloration dark brown, with gray pruinosity (Fig. 2b). Pronotum with light hairs. Prescutum with four longitudinal brown bands; lateral bands short, median bands distinctly separated. Anepisternum, katepisternum and anepimeron with dense, gray pruinosity. Scutum, scutellum, laterotergite and mediotergite unicolorous, dark brown. Coxae brown, with light hairs. Trochanters yellowish, with light hairs. Proximal part (ca. two thirds) of femora yellowish, turning dark brown toward tips. Tibiae and tarsi dark brown, spur formula 1:2:2. Tarsal claws smooth. Legs covered with dark brown – black bristles. Stem of halter yellowish, knobs infuscated. Wings with marmorate pattern, length (n= 5) 11.9 mm (11–12.6 mm), venation as in Fig. 2c. R1+2 is variable, reach or not reach Costa. Wing cells c and sc yellowish, other cells brown tinged (see Figs. 2b, 2c). Pterostigma distinct. Abdomen yellowish brown, with a narrow dorsal stripe (Fig. 2b). Hypopygium (Fig. 3a) dark brown. Caudal margin of 9th tergite with a median notch, bearing no tooth or other elevated structures (Figs. 3g–h). Caudal margin of 9th tergite oblique (Finnish specimens) or almost horizontal, truncated (Russian specimens) (Figs. 3g–h). Outer gonostylus narrow, about as long as inner gonostylus, slightly bent sub-basally (Figs. 3b, d). Lower beak of inner gonostylus apically rounded, black. Beak of inner gonostylus rather narrow and elongated in lateral view (Figs. 3b, d), tip roundish and proximal margin oblique, notched in posterior view (Fig. 3c). Outer basal lobe of inner gonostylus with 3–4 stout black spines. Aedeagal guide as in Figs. 3e–f. Sperm pump hairy between posterior immovable apodemes, apex of aedeagus pointed (Figs. 3i–j).
Female. Wing length (n=3) 12.8 mm (12.5–13.5 mm), body length (n=3) 12.3 mm (12–13 mm). Generally similar to male (Fig. 2e). Antenna short (2.4 mm), not extending to wing base (Fig. 2d). The wing’s marmorate pattern more intensive than in male (Fig. 2f). Ovipositor (Figs. 4a, b) elongate, similar to that of most other tipulines; 8th tergite dark brown, 9th tergite narrow dull dark brown, 10th tergite shining chestnut brown. 8th sternite dull dark brown anteriorly, grading to shining yellow posteriorly. Cerci narrow, yellow, slightly longer than 10th tergite. Hypogynial valves yellow, reaching mid-length of cerci, relatively wide, gradually narrowing (Fig. 4c).
Etymology
The species epithet is from reconditus (Latin, adjective) meaning hidden, concealed. This word refers to the rarity and apparent low detectability of the new species, so far known only from two sites in the Palaearctic region.
Distribution and ecology
Tipula (Pterelachisus) recondita Pilipenko & Salmela, sp. n. is known from North Europe (Finland) and Asia, Russian Far East. The Finnish collecting site in Kittilä, Iso Mustavaara, is a state-owned Nature Reserve (Lehtojensuojelualue), included in the Natura2000 network of conservation areas. It is part of the biogeographical province of Lkoc (Lapponia kemensis pars occidentalis) and lies in the North boreal vegetation zone. The collecting site is an old-growth mixed forest, dominated by birch (Betula pubescens), goat willow (Salix caprea) and Norway spruce (Picea abies), with scattered aspen (Populus tremula) trees. Lower vegetation is characterized by herbs and shrubs such as Calypso bulbosa, Daphne mezereum, Actaea erythrocarpa, Ribes spicatum, Filipendula ulmaria and Geranium sylvaticum. Decaying trees, especially goat willow and birch, are abundant in the site. The Russian collecting site is located in the Kedrovaya Pad’ Nature Reserve, within the temperate broadleaf and mixed forest zone, in an oak forest (Quercus mongolica) growing on limestone outcrops on the southern slope of a mountain range. Lower vegetation is characterized by Lespedeza bicolor, Spodiopogon sibirieus, Astra ageratoides, Carex siderosticta, Artemisia keiskeana, Lathyrus davidii and Calamagrostis brachytricha.
Discussion
Tipula (Pterelachisus) recondita Pilipenko & Salmela, sp. n. is rather easily distinguished from other Holarctic Tipula (Pterelachisus) species. The new species is distinctive in characters of the male hypopygium, especially that of the 9th tergite. There are several Tipula (Pterelachsus) species with a U-shaped median notch or an emargination in the caudal margin of the tergite, but usually having a tooth or other elevated structures at the mid-point (e.g. Tipula (Pterelachisus) angulata Loew [Alexander 1919[2], p. 984, Salmela and Autio 2007[3], p. 55], Tipula (Pterelachisus) varipennis Meigen [Savchenko 1964[4], p. 56], Tipula (Pterelachisus) imitator Alexander [Alexander 1953[5], Plate 1], Tipula (Pterelachisus) resupina Alexander [Alexander 1935[6], Plate 2]); the new species is peculiar having no such structures in the 9th tergite.
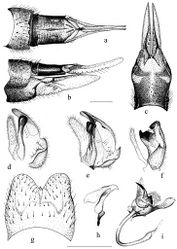
Morphologically the new species is perhaps the closest to two Palaearctic species, namely Tipula (Pterelachisus) imitator and Tipula (Pterelachisus) pauli. The former species has a median notch in 9th tergite, but also a distinct tooth at the midpoint (Fig. 4g); the outer basal lobe of inner gonostylus bears one conspicuous black spine, not 3–4 smaller ones (Fig. 4e). For other differences, see Figures 4f, h, i. Tipula pauli also has a median notch in 9th tergite and a small but discernible tooth in the midpoint; the lower beak of inner gonostylus is roundish and black, but the outer basal lobe bears no stout, black spines (Fig. 4d). Tipula (Pterelachisus) imitator is known from Japan and Kuril Islands and Tipula (Pterelachisus) pauli from Europe, Altay and Russian Far East (Oosterbroek 2012, V. Pilipenko pers. obs.).
Based on COI divergence, the new species is apparently rather isolated from the members of the subgenus Pterelachisus (Fig. 1). Among the other species vs. the new species, interspecific distances varied from 5.3 % (Tipula winthemi Lackschewitz) to 16.1 % (Tipula laetibasis Alexander). Mean of the minimum interspecific distances was 8.8 %. According to K2P divergence, the new species is closest to Tipula winthemi (5.3 %), Tipula jutlandica Nielsen (5.5 %), Tipula stenostyla Savchenko (6.6 %) and Tipula pauli (6.8 %); distances between the other species range from 7.4 to 16.1 %. In other words, no very close relatives were present in the pair-wise comparisons of COI sequences. For example, much shorter interspecific K2P distances were found between Tipula varipennis/Tipula pseudovariipennis (1.5 %), Tipula mutila/Tipula wahlgreni (2.2 %), Tipula stenostyla/Tipula winthemi (3.7 %). However, it must be emphasized that Tipula imitator was not included in COI analysis, due to the lack of fresh material. Given to the morphological similarity of the new species and Tipula imitator, it is likely that their barcoding distances would be similar to those three comparisons given above.
There are some morphological differences (9th tergite, inner gonostylus) between Finnish and Russian specimens, perhaps due to the long distance and lack of gene flow between the populations. These differences, however, are here considered to be intraspecific variation. Very small K2P divergence of COI gene (0.2 %) between Finnish and Russian specimens also substantiates the presence of one widespread, but disjunct, species. In rare cases (see Burns et al. 2007[7]) differences of only one to three nucleotides may be observed between otherwise (for example morphologically and ecologically) distinct species. However, in this case we were able to produce 524 bp of high quality sequence, instead of <400 as in the problematic cases of Burns et al. (2007)[7]. Moreover, the known biologies of the Finnish and Russian individuals seem alike. To say more of the COI variation, it would be essential to collect more individuals which is rather difficult, given the rarity of the species.
The new species is most probably a very rare tipulid. Despite the rather long tradition of crane fly taxonomy and faunistics in North Europe, this species has hitherto remained unnoticed. One of the authors (JS) has within 12 years identified some 70 000 crane flies from a Finnish Malaise trapping material consisting of 476 sampling sites and ca. 1670 Malaise trapping months. Thus, despite this relatively large sampling effort, only three specimens from a single locality have been caught. The true range of the species is Palaearctic, whether disjunct or not remains to be seen. In Northwestern Europe the species is likely to occur in the north boreal zone (for further information on boreal ecoregions or vegetation zones, see e.g. Ahti et al. 1968[8]). Tipula (Pterelachisus) recondita Pilipenko & Salmela, sp. n. may be confined to old-growth forests, and its rarity is perhaps due to the narrow habitat niche. On the other hand, the new species may be hard to collect using traditional methods. Larval associations of this species are unknown, but some Tipula (Pterelachisus) species are saproxylic, i.e. dependent on decaying trees. Such species are e.g. Tipula (Pterelachisus) pseudoirrorata Goetghebuer and Tipula (Pterelachisus) stenostyla Savchenko (Salmela 2009[9]), both of them also encountered in the type locality.
To our regret we were not able to examine the holotype male of Tipula imitator (D. Furth, pers. comm.). Description of that species was based on a single male specimen (Alexander 1953[5]). We have however examined other material (two male specimens, see above) that very likely represents Tipula imitator. Despite morphological similarity of Tipula (Pterelachisus) recondita Pilipenko & Salmela, sp. n. and Tipula imitator, we are confident that these are separate taxa, due to the differences in the structure of male hypopygium.
Original Description
- Pilipenko, V; Salmela, J; Vesterinen, E; 2012: Description and DNA barcoding of Tipula ( Pterelachisus) recondita sp. n. from the Palaearctic region (Diptera, Tipulidae) ZooKeys, 192: 51-65. doi
Other References
- ↑ Salmela J (2011) Annotated list of Finnish crane flies (Diptera: Limoniidae, Tipulidae, Pediciidae & Cylindrotomidae). Entomologica Fennica 22: 219-242.
- ↑ Alexander C (1919) The crane-flies of New York. Part I. Distribution and taxonomy of the adult flies. Memoirs, Cornell University Agricultural Experiment Station 25: 767-993.
- ↑ Salmela J, Autio O (2007) Redescription of Tipula octomaculata Savchenko, with notes on related Holarctic species (Diptera, Tipulidae). Zootaxa 1527: 53-58.
- ↑ Savchenko E (1964) Crane-flies (Diptera, Tipulidae), Subfam. Tipulinae, Genus Tipula L., 2. Fauna USSR, N.S. 89, Nasekomye Dvukrylye [Diptera], 2(4): 1–503 [in Russian].
- ↑ 5.0 5.1 Alexander C (1953) Records and descriptions of Japanese Tipulidae (Diptera). Part I. The crane-flies of Shikoku. I. Philippine Journal of Science 82: 21-75.
- ↑ Alexander C (1935) New or little-known Tipulidae from eastern Asia (Diptera). XXIV. Philippine Journal of Science 56: 525-562.
- ↑ 7.0 7.1 Burns J, Janzen D, Hajibabaei M, Hallwachs W, Hebert P (2007) DNA barcodes of closely related (but morphologically and ecologically distinct) species of skipper butterflies (Hesperiidae) can differ by only one to three nucleotides. Journal of the Lepidopterists’ Society 63: 138-153.
- ↑ Ahti T, Hämet-Ahti L, Jalas J (1968) Vegetation zones and their sections in northwestern Europe. Annales Botanici Fennici 5: 169-211.
- ↑ Salmela J (2009) The subgenus Tipula (Pterelachisus) in Finland (Diptera, Tipulidae) – species and biogeographic analysis. Zoosymposia 3: 255-271.
Images
|