Siderasis
Contents
- 1 Taxonavigation
- 2 Name
- 3 Type species
- 4 Description
- 5 Etymology
- 6 Habitat, distribution and ecology
- 7 Biogeography
- 8 Growth form and leaf morphology
- 9 Inflorescence morphology
- 10 Floral symmetry
- 11 Androecium and gynoecium morphology
- 12 Fruit and seed morphology
- 13 Reproductive biology
- 14 Taxon Treatment
- 15 Images
- 16 Other References
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Pellegrini2017PhytoKeys, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Pellegrini2017PhytoKeys">{{Citation See also the citation download page at the journal. |
Ordo: Commelinales
Familia: Commelinaceae
Name
Siderasis Raf., Fl. Tellur. 3: 67. 1837, emend. M.Pell. & Faden – Wikispecies link – Pensoft Profile
- Pyrrheima Hassk., Flora 52: 366. 1869, nom. illeg. Type species (designated here). P. loddigesii Hassk., nom. illeg. [≡ S. fuscata (Lodd.) H.E.Moore].
Type species
Siderasis acaulis Raf. [≡ S. fuscata (Lodd.) H.E.Moore].
Description
Herbs or vines , perennial, with a definite base, terrestrial or rupicolous. Roots thin, fibrous, sometimes forming terminal, small, fusiform to oblongoid tubers. Rhizomes present or not, if present short, shallowly to deeply buried in the ground, rarely only covered by leaf litter. Subterraneous stems present or not, when present buried deep in the soil, unbranched, produced directly from the short rhizome; internodes moderately elongate to elongate. Aerial stems with determinate or indeterminate growth, elongated or short to inconspicuous, densely branched or unbranched, when densely branched primary shoot determinate or not, when present secondary shoots determinate; internodes inconspicuous to weakly elongate, or elongate; flagelliform-shoots (ramets) present or not, if present produced after the fertile period, forming a new rosette at the apex, axillary, unbranched, internodes elongate. Leaves spirally-alternate or distichously-alternate, congested at the apex of the stems forming a rosette or evenly distributed along the secondary branches, sessile to subpetiolate or petiolate, sheathing at the base, ptyxis involute; blades membranous to chartaceous or succulent, base symmetric or slightly to completely asymmetric, margins slightly revolute to flat, apex curved or straight. Synflorescence composed of a solitary main florescence or with 1–7 coflorescences. Main florescence (inflorescence) a thyrse, terminal or apparently so, rarely axillary, a many-branched, pedunculate thyrse, with alternate cincinni or reduced to a solitary pedunculate cincinnus; basal bract sessile or amplexicaulous or sheathing; cincinni bracts sessile or amplexicaulous; cincinni pedunculate, 1–many-flowered; bracteoles present or not. Flowers bisexual or staminate, actinomorphic or zygomorphic, chasmogamous, flat, pedicellate or sessile; pedicels erect during pre-anthesis and anthesis, erect or deflexed post-anthesis, generally elongating in fruit; sepals 3, unequal, free, membranous or fleshy, persistent and accrescent in fruit, the uppermost external, broader than the others, sometimes also shorter than the others; petals 3, deliquescent, free, margins entire to irregularly lacerated, glabrous, rarely ciliated with non-moniliform hairs, apex entire to irregularly lacerated, subequal, the lowermost either broader or longer than the others; stamens 6, equal or unequal, straight or curved upwards, filaments free, glabrous, straight or sigmoid, anthers dorsifixed, extrorsely rimose, anther sacs semicircular, divergent, pollen white, connectives expanded, quadrangular to rectangular; ovary sessile, globose to broadly oblongoid to ellipsoid in outline, trigonous with obtuse to round angles in cross-section, densely hirsute or lanate or velutine, 3-locular, locules equal, 3–6-ovulate, ovules hemianatropous, biseriate to partially uniseriate; style terminal, straight or curved upwards; stigma annular-truncate or annular-capitate, marginally papillate leaving the stylar canal evident, papillae unicellular. Capsules loculicidal, thick-walled, 3-valved, globose or subglobose to broadly ellipsoid to broadly oblongoid to oblongoid in outline, trigonous with obtuse to round angles in cross-section, smooth to sparsely reticulate, apiculate due to persistent style base. Seeds (1–)3–6 per locule, arillate, obconic to ellipsoid, dorsiventrally compressed, ventrally slightly flattened or with a mild ridge, testa foveolate or rugose; hilum C-shaped, in a shallow depression; embryotega semidorsal or semilateral, relatively inconspicuous, without a prominent apicule; aril cream-colored to hyaline, slightly to completely translucent, thick or inconspicuous.
Etymology
Siderasis was named in allusion to the peculiar red to bright-red hairs that cover almost the entire plant, but especially the leaves. However, only S. fuscata possesses the aforementioned hairs, and all of the remaining species possess leaf blades covered by hyaline to light brown, rarely rusty hairs.
Habitat, distribution and ecology
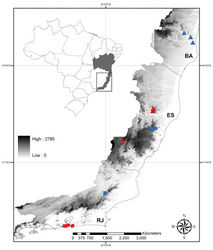
Siderasis is endemic to the Atlantic Forest domain in coastal Brazil, occurring in the states of Bahia, Espírito Santo, and Rio de Janeiro (Fig. 2). More specifically, Siderasis is restricted to the Central Corridor of the Atlantic Forest, growing in remnants of semideciduous forests associated with inselbergs, between 90–1350 m above sea level. The genus is composed exclusively by microendemic species distributed in very small and fragmented subpopulations, susceptible to deforestation and illegal collection of specimens for ornamental purposes.
Biogeography
Since most phylogenies for Commelinaceae corroborate the paraphyly of Dichorisandrinae (Evans et al. 2000[1], 2003[2]; Hardy 2001[3]; Wade et al. 2006[4]; Zuiderveen et al. 2011[5]; Hertweck and Pires 2014[6]; Pellegrini et al., in prep.), we can hypothesize on the independent diversification of these lineages from a biogeographical point of view. The clade composed by Cochliostema, Geogenanthus, and Plowmanianthus is consistently recovered as the second lineage to diverge in tribe Tradescantieae, following the diversion of subtribe Streptoliriinae (Evans et al. 2003[2]; Wade et al. 2006[4]; Zuiderveen et al. 2011[5]; Hertweck and Pires 2014[6]; Pellegrini et al., in prep.). The ancestor of this lineage probably originated in the Amazon Basin, and posteriorly diversified in the Guyana Shield, northern Andes, and Central America reaching Costa Rica (Hardy 2001[3]; Hardy and Faden 2004[7]). On the other hand, the clade composed by Dichorisandra and Siderasis is recovered as the third lineage to diverge in Tradescantieae (Evans et al. 2003[2]; Wade et al. 2006[4]; Zuiderveen et al. 2011[5]; Hertweck and Pires 2014[6]; Pellegrini et al., in prep.). The ancestor of this clade probably originated and diversified in the Atlantic Forest domain, since it is the center of diversity of both genera. Subsequently, the ancestors of various Dichorisandra lineages might have dispersed, more than once, diversifying in the Amazon Basin through gallery forests in the Cerrado domain.
Growth form and leaf morphology
Siderasis possesses two clearly differentiated growth patterns: (1) rosette herbs, generally with very short internodes, and spirally-alternate, symmetrical leaves (Fig. 3A–B); (2) climbing vines, with elongated internodes, and distichously-alternate, asymmetrical leaves (Fig. 3C–D). The rosette habit has hitherto been the only one recognized in the genus. Faden (1998)[8] mentioned the existence of a climbing species in the genus, but due to the synoptic nature of that publication, no further remarks were made on the subject. The climbing habit is relatively uncommon in the family, but found in the closely related Dichorisandra. However, in Dichorisandra the plants tend to lean on nearby trees and shrubs, later producing pendant branches, or even growing completely intertwined with more robust shrubs (Fig. 3E–F). In Siderasis, the primary branch grows at the base of a tree (Fig. 3C), posteriorly spirally ascending around the trunk, and finally producing the flowering secondary branches (Fig. 3D). In the remaining genera of Dichorisandrinae, growth form is stable, with almost no variation within each genus. In Cochliostema, the plants tend to be tank-forming rosette herbs, but creeping individuals are also known in C. velutinum Read (Hardy 2001[3]). In Geogenanthus, the plants always possess a dracaenoid habit, with leaves congested at the apex (Hardy 2001[3]). In Plowmanianthus, the plants are always rosette herbs with very short stems (Hardy and Faden 2004[7]).
Considerable variation in leaf morphology occurs in Siderasis, with leaves ranging from: (1) sessile to subpetiolate (Fig. 3B–D); (2) truly petiolate, as in S. fuscata (Fig. 3A, 8C). Truly petiolate leaves are extremely rare in Commelinaceae, being recorded only in a handful of species restricted to the peculiar-looking subtribe Streptoliriinae, mostly comprised of vining plants (Pellegrini and Faden, pers. observ.). Phyllotaxy in Siderasis can range from distichous to spirally-alternate, the arrangement being correlated to symmetry of the leaf blades.
Inflorescence morphology
In all Dichorisandra and two species of Siderasis (i.e. S. spectabilis and S. zorzanellii), the main florescence is a many-branched, pedunculate, terminal or axillary thyrse with alternate cincinni, each cincinnus being multi-flowered. In the remaining species of Siderasis (i.e. S. albofasciata, S. almeidae, S. fuscata and S. medusoides), the main florescence is composed of a thyrse reduced to a solitary cincinnus, as described in Pellegrini (2017[9]; Fig. 4A). These reduced thyrsi are arranged into a synflorescence that may contain up to seven coflorescences. The center of the mature Siderasis rosette may contain several terminal or apparently terminal synflorescences. In Dichorisandra and the two climbing species of Siderasis, the main axis of the inflorescence is usually well developed, thus producing a typical looking thyrse (Fig. 4B). Nevertheless, the inflorescences may also be extremely reduced in some species (i.e. D. acaulis group), due to the shortening of the inflorescence’s internodes (Pellegrini and Almeida 2016[10]). The cincinni are also very short (i.e. sessile to subsessile), being enclosed by the leaf sheaths and not obvious at first glance (Pellegrini and Almeida 2016[10]). The flowers are peculiarly long-pedicellate, giving the impression that all flowers emerge directly from the apex of the stems (Pellegrini and Almeida 2016[10]; Fig. 4C). Despite the extreme reduction and superficial similarity, this inflorescence pattern differs from the one found in the rosette species of Siderasis, since it still is a many-branched thyrse. In Plowmanianthus the main florescence is also reduced to a solitary cincinnus. Nonetheless, coflorescences only develop after the main florescence has failed to develop or set fruit, and the cincinni from the primary and secondary thyrsi are morphologically distinct (Hardy and Faden 2004[7]). In Geogenanthus the inflorescences are always born at the base of the plant, near the ground. Aside from that, the main florescence is a pedunculate, fascicle-like thyrse, with (1–)2–4–several alternate cincinni (Hardy 2001[3]). Finally, in Cochliostema the main florescence is a many-branched, pedunculate, axillary thyrse, with alternate to verticillate cincinni, each cincinnus being multi-flowered and subtended by showy and cucullate spathaceous bracts (Hardy and Stevenson 2000[11]; Hardy 2001[3]).
Floral symmetry
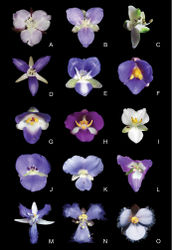
Two distinct floral patterns can be observed in different species of Siderasis: (1) flowers are always bisexual, actinomorphic, having 6 equal stamens arranged cyclically around the ovary, with straight filaments (Fig. 1A–B); (2) flowers bisexual or staminate, zygomorphic, having 6 unequal stamens curved upwards, with sigmoid filaments (Fig. 1C). Furthermore, in the zygomorphic staminate flowers, the lower antepetalous stamen is longer, and is arranged and curved in the same way as the style in bisexual flowers. The first flower morph is very similar to that found in the D. acaulis group (Pellegrini and Almeida 2016[10]; Fig. 1D), while the second is equivalent to that of the D. hexandra and D. incurva groups (Fig. 1E, I). In Dichorisandra, flower symmetry is generally influenced by the positioning of the stamens, rather than by the relationship of stamens and staminodes. Actinomorphic flowers can be found not only in the D. acaulis group (Fig. 1D), but also in a group of still-undescribed species from the Guyana Shield (Faden and Pellegrini, pers. observ.). In all remaining species groups in Dichorisandra, the flowers are clearly zygomorphic, either due to the number of stamens, their size and/or position. In the D. thyrsiflora group, the androecium is generally composed of six fertile stamens, four of them curved towards the center of the flower, and the two lower lateral ones curved towards their opposing sides (Fig. 1F). An exception can be noticed in D. paranaënsis D.Maia et al. (Fig. 1G) and D. nana Aona & M.C.E.Amaral (Fig. 1H). In D. paranaënsis the stamens are curved upwards, varying from five fertile stamens with a staminode (present or not) to six fertile stamens, and introrsely rimose anthers. On the other hand, in D. nana the six fertile stamens are curved upwards, and possess poricidal anthers. In the D. incurva (Fig. 1I), D. penduliflora (Fig. 1J), D. leucophthalmos (Fig. 1K), and D. radicalis groups (Fig. 1L), the androecium is composed of five stamens (generally with an upper staminode; notice the filiform staminode in Fig. 1L), rarely six fertile stamens, curved upwards, and with introrsely rimose anthers. In the D. incurva and D. leucophthalmos groups, the anthers are always yellow (Figs 1I, K), while in the D. pendulifora and D. radicalis groups, the anthers are white, generally with the anther sacs partially to totally colored in blue, pink or purple (Figs 1J, L). The remaining genera of Dichorisandrinae possess strongly zygomorphic flowers, especially due to the position and/or number of stamens: (1) 5–6 dimorphic, free and fertile stamens in Geogenanthus (Hardy 2001[3]; Fig. 1N); (2) 3 stamens in the upper side of the flower, fused in a hood-like structure, and 3 lower staminodes (the middle one microscopic) in Cochliostema (Hardy 2001[3]; Fig. 1M); (3) and 3 free to partially fused stamens in the upper side of the flower, and 3 lower staminodes (generally all of them microscopic) in Plowmanianthus (Hardy and Faden 2004[7]; Fig. 1O).
Androecium and gynoecium morphology
The anthers in Siderasis are dorsifixed, with extrorsely rimose dehiscence, two times wider than long, three to four times shorter than the filaments, with semicircular, divergent anthers sacs, and expanded connectives (Fig. 1A–C). In Dichorisandra the anthers are basifixed, with poricidal or introrsely rimose (but functionally poricidal) dehiscence, three to four times longer than wide, and three to four times longer than the filaments, rarely equal to the filaments, with elongate, parallel anther sacs, and inconspicuous connectives (Aona 2008[12]; Figs 1D–L). In Cochliostema, Geogenanthus and Plowmanianthus the anthers vary from dorsifixed to basifixed, with extrorsely rimose dehiscence, as wide as long to two times shorter than the filaments, with semicircular to spirally-coiled, appressed anther sacs, and inconspicuous connectives (Hardy and Stevenson 2000[11]; Hardy 2001[3]; Hardy and Faden 2004[7]; Figs 1M–O).
The gynoecium is fairly homogeneous in Dichorisandrinae s.l., with all genera having sessile, 3-locular ovaries, with all locules fertile, ovules hemianatropous, biseriate to partially uniseriate, style terminal, straight or bent at the apex, stigma annular-truncate to annular-capitate, peripherally ciliate with moniliform hairs (i.e. Cochliostema and Plowmanianthus) or not (i.e. Dichorisandra, Geogenanthus and Siderasis). In Siderasis, the stigmatic papillae are unicellular, and restricted to the margins of the stigma, leaving the stylar canal evident (Owens and Kimmins 1981[13]). On the other hand, in Dichorisandra, the stigmatic papillae are multicellular, and evenly distributed on the stigma, completely concealing the stylar canal (Owens and Kimmins 1981[13]).
Fruit and seed morphology
The capsules of Dichorisandra and Siderasis can be differentiated from capsules of other Commelinaceae by their thick and tough walls. In Commelinaceae the fruits are commonly (1–)2–3-locular, thin walled, septicidal capsules (Faden 1998[8]). Dichorisandra and Siderasis possess 3-locular, 3-valvar capsules, and arillate seeds. The aril in Dichorisandra is generally opaque (rarely hyaline), usually thick (rarely inconspicuous), and colored from white to grayish or bright orange (rarely colorless) (Fig. 5A–B). Whereas the aril in Siderasis can be hyaline to slightly hyaline, inconspicuous or thick, and cream-colored to colorless (Fig. 5C–D). The seeds in both genera are very similar in gross morphology, varying in shape from obconic to ellipsoid to quadrangular; in ornamentation from foveolate to scrobiculate to rugose, with a semilateral to semidorsal embryotega, and with a C-shaped hilum. In Cochliostema, Geogenanthus and Plowmanianthus the capsules are thin-walled, 3-locular, 3-valvar, and with exarillate seeds. In Cochliostema the capsules are narrowly cylindrical, and the seeds vary from subcylindrical to narrowly oblongoid, with a smooth testa that becomes sticky when hydrated, semidorsal embryotega, and a linear hilum with curved edges. In Geogenanthus and Plowmanianthus the capsules are fusiform to ellipsoid, the seeds range from reniform to ellipsoid, with rugose to foveolate, farinose testa, lateral embryotega, and a C-shaped hilum (Hardy 2001[3]; Hardy and Faden 2004[7]).
In Dichorisandra and Siderasis capsule and seed morphology differences may have great taxonomic potential. In Dichorisandra, many of the aforementioned species groups display characteristic capsule and seed morphology, as exemplified in the D. acaulis group by Pellegrini and Almeida (2016)[10]. In the D. thyrsiflora group, capsule morphology can easily differentiate most known species, based on shape, coloration, texture and pubescence (Pellegrini, pers. observ.). In Siderasis, capsule morphology shows a similar potential, with the fruits of S. zorzanellii being completely deviant in shape, texture and pubescence from the remaining species. Unfortunately, since the fruits of S. spectabilis are still unknown, it is impossible to know if this change in capsule morphology is correlated to the change in habit from rosette to vining herbs. Siderasis fuscata possesses unique seed morphology, being the only known species with an inconspicuous and hyaline aril, testa light gray to gray, and foveolate. Field expeditions focused on collecting fruit and seed samples of all species of Siderasis could be of great taxonomic value. It is possible that most, if not all, presently accepted species could be differentiated based exclusively on fruit and seed morphology.
Reproductive biology
Little is known regarding the floral biology of subtribe Dichorisandrinae, although this subtribe possesses the greatest range in inflorescence architecture and floral patterns in the family. In Siderasis the anthers are always extrorsely rimose, but apart from the floral specialization (i.e. zygomorphic, bisexual or staminate flowers, and unequal and sigmoid stamens) in the two climbing species and the petals with margins ciliated with non-moniliform hairs, a character unique in the family, in S. spectabilis, the flowers are relatively unspecialized. Dichorisandra possesses a wide variation in flower morphology and androecium arrangement. Its flowers can range from actinomorphic to zygomorphic, the stamens can vary from (5–)6, sometimes with the upper stamen reduced to a staminode in some species. The filaments can be either straight, slightly sigmoid or slightly twisted depending on their position in the flower, while the anthers can be introrsely rimose and functionally poricidal or truly poricidal (Aona 2008[12]; Fig. 1D–L). On the other hand, in Cochliostema, Geogenanthus and Plowmanianthus, the flowers are highly specialized, being zygomorphic (in all genera), scented (in Cochliostema), with a high frequency of cleistogamous flowers (in Plowmanianthus), petals and stigma fringed with moniliform hairs (fringed petals in all genera, stigma fringed exclusively in Cochliostema and Plowmanianthus), filaments bearded with moniliform hairs (in all genera), functionally poricidal androecium (in Cochliostema, due to the hood-like structure enclosing the anthers), and curved to spirally-coiled anthers (in all genera) (Hardy and Stevenson 2000[11]; Hardy 2001[3]; Hardy and Faden 2004[7]; Fig. 1M–O). Only three species of Dichorisandra have had their reproductive biology investigated, presenting typical buzz-pollination, performed by bumblebees (Apidae) and/or sweatbees (Halictidae) (D. thyrsiflora, Boaventura and Matthes 1987[14]; D. hexandra and D. incurva, Sigrist and Sazima 2015[15]). Information regarding flower visitation in Cochliostema, Geogenanthus, and Plowmanianthus is completely lacking from the available literature. During our field studies and while observing the Siderasis specimens grown at the greenhouse of Jardim Botânico do Rio de Janeiro, the first author has observed flowers of S. albofasciata, S. almeidae, and S. fuscata being visited by stingless honey bees (Apidae, tribe Meliponini). Siderasis medusoides was not seen in the field, but high-resolution photographs sent by one of the collectors clearly show several small ants walking around the flowers and cincinni (Fig. 9C). The bees might either represent pollen robbers or potential pollinators, but the presence of the ants is hard to explain, since nectaries are unknown for Commelinaceae (Faden 1992[16], 1998[8]). Further studies on the reproductive biology of Siderasis are clearly needed.
Aside from the peculiar floral diversity, Dichorisandrinae s.l. has two genera (out of five) and the majority of species in the family with arillate seeds (Pellegrini 2017[9]). Nonetheless, no study has ever focused on vector-mediated (i.e. zoo-choric) seed dispersal in the family. In Dichorisandra, the seeds in the D. hexandra group are most certainly dispersed by birds (Faden 1992[16]), due to the plants vining habit (Fig. 3E), which help in displaying the seeds, covered by an orange to bright orange, thick and opaque aril (Fig. 5B). The seeds in the D. thyrsiflora group are covered by a thick and opaque, white to cream-colored aril (Fig. 5A), and are generally easy to see in the field, due to the plants high stature (Pellegrini pers. observ.). Nonetheless, these species lack the characteristic colors that are generally associated with bird pollination/dispersal (i.e. pink, red, orange and yellow; Fleming and Estrada 1993[17]), always present in the D. hexandra group. The species in the D. acaulis group possess seeds also covered by a thick and opaque, white aril, lacking the visual attraction associated with bird dispersal, and also lack an elevated display, since they are always shorter than 1 m long (Pellegrini and Almeida 2016[10]). These seeds might be dispersed by ants, or by small terrestrial vertebrates (e.g. small rodents), instead of being dispersed by birds, as hypothesized for other species of Dichorisandra. The seeds from the rosette species of Siderasis have similar morphological and ecological features to the species from the D. acaulis group. These species also have small stature and seeds with hyaline and inconspicuous, or cream-colored, slightly translucent, thick arils (Fig. 5C–D), being most probably dispersed by animals similar to the ones dispersing the seeds of the species in the D. acaulis group.
From a phylogenetic point of view, it seems that vector-mediated seed dispersal has evolved several times in the family: (1) arillate seeds are recorded for Dichorisandra and Siderasis in Dichorisandrinae, Amischotolype Hassk., Coleotrype C.B.Clarke and Porandra Hong in Coleotrypinae (Pellegrini 2017[9]), and Spatholirion Ridl. in Streptoliriinae (Thitimetharoch 2004[18]); (2) appendaged seeds are recorded for at least two separate lineages in tribe Commelineae (i.e. some species of Commelina L. and Murdannia Royle; Pellegrini et al. 2016[19]); (3) truly fleshy fruits are known only from Palisota Rchb. ex Endl. (Faden 1998[8]); (4) in Tradescantia zanonia (L.) Sw. the fleshy sepals cover the indehiscent fruit at post-anthesis, producing a sweet and atro-vinaceous berry-like fruit, dispersed by birds (Pellegrini, obs. pers.); (5) in Pollia Thunb. the fruits are dry, crustaceous and indehiscent, and due to their vibrant colors (metallic blue to shiny black) mimic real berries (Faden 1978[20]); (6) in some Commelina (i.e. the species originally placed under Phaeosphaerion Hassk. and Commelinopsis Pichon) the fruits are morphologically similar to those of Pollia, being also crustaceous, but either dehiscent or indehiscent (Faden and Hunt 1987[21]); (7) and sticky capsules covered by a mixture of hook and minute glandular hairs, in Rhopalephora Hassk. (Pellegrini et al., in prep.). Nonetheless, further investigations are needed to better understand the ecology and evolution of vector-mediated seed dispersal in Commelinaceae.
Taxon Treatment
- Pellegrini, M; Faden, R; 2017: Recircumscription and taxonomic revision of Siderasis, with comments on the systematics of subtribe Dichorisandrinae (Commelinaceae) PhytoKeys, (83): 1-41. doi
Images
|
Other References
- ↑ Evans T, Faden R, Simpson M, Sytsma K (2000) Phylogenetic relationships in the Commelinaceae: I. A cladistic analysis of morphological data. Systematic Botany 25: 668–691. https://doi.org/10.2307/2666727
- ↑ 2.0 2.1 2.2 Evans T, Sytsma K, Faden R, Givnish T (2003) Phylogenetic relationships in the Commelinaceae: II. A cladistic analysis of rbcL sequences and morphology. Systematic Botany 28: 270–292.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 Hardy C (2001) Systematics of Cochliostema, Geogenanthus, and an undescribed genus in the spiderwort family, Commelinaceae. PhD Thesis, Cornell University, Ithaca, New York.
- ↑ 4.0 4.1 4.2 Wade D, Evans T, Faden R (2006) Subtribal relationships in tribe Tradescantieae (Commelinaceae) based on molecular and morphological data. Aliso 22(1): 520–526. https://doi.org/10.5642/aliso.20062201.40
- ↑ 5.0 5.1 5.2 Zuiderveen G, Evans T, Faden R (2011) A phylogenetic analysis of the African plant genus Palisota (family Commelinaceae) based on chloroplast DNA sequences. Grand Valley State University, Honors Projects: Paper 65. http://scholarworks.gvsu.edu/honorsprojects/65
- ↑ 6.0 6.1 6.2 Hertweck K, Pires J (2014) Systematics and evolution of inflorescence structure in the Tradescantia alliance (Commelinaceae). Systematic Botany 39(1): 105–116. https://doi.org/10.1600/036364414X677991
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Hardy C, Faden R (2004) Plowmanianthus, a new genus of Commelinaceae with five new species from Tropical America. Systematic Botany 29(2): 316–333. https://doi.org/10.1600/036364404774195511
- ↑ 8.0 8.1 8.2 8.3 Faden R (1998) Commelinaceae. In: Kubitzki K (Ed.) The families and genera of vascular plants, vol. 4. Springer Verlag. Berlin, 109–128. https://doi.org/10.1007/978-3-662-03531-3_12
- ↑ 9.0 9.1 9.2 9.3 Pellegrini M (2017) Siderasis albofasciata sp. nov. (Commelinaceae), endemic to the state of Espírito Santo, Brazil, and the typification of S. fuscata. Nordic Journal of Botany 35(1): 29–37. https://doi.org/10.1111/njb.01267
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Pellegrini M, Almeida R (2016) Rediscovery, identity and typification of Dichorisandra picta (Commelinaceae) and comments on the short-stemmed Dichorisandra species. Phytotaxa 245(2): 107–118. https://doi.org/10.11646/phytotaxa.245.2.2
- ↑ 11.0 11.1 11.2 Hardy C, Stevenson D (2000) Development of the gametophytes, flower, and floral vasculature in Cochliostema odoratissimum (Commelinaceae). Botanical Journal of the Linnean Society 134: 131–157. https://doi.org/10.1111/j.1095-8339.2000.tb02348.x
- ↑ 12.0 12.1 Aona L (2008) Revisão taxonômica e análise cladística do gênero Dichorisandra J.C.Mikan (Commelinaceae). PhD Thesis, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, São Paulo.
- ↑ 13.0 13.1 Owens S, Kimmins F (1981) Stigma morphology in Commelinaceae. Annals of Botany 47(6): 771–783. https://doi.org/10.1093/oxfordjournals.aob.a086076
- ↑ Boaventura Y, Matthes L (1987) Aspectos da biologia da reprodução em plantas ornamentais cultivadas no estado de São Paulo I: Dichorisandra thyrsiflora J.C.Mikan (Commelinaceae). Acta Botânica Brasílica 1(2): 189–199. https://doi.org/10.1590/S0102-33061987000200007
- ↑ Sigrist M, Sazima M (2015) Phenology, reproductive biology and diversity of buzzing bees of sympatric Dichorisandra species (Commelinaceae): breeding system and performance of pollinators. Plant Systematics and Evolution 301(3): 1005–1015. https://doi.org/10.1007/s00606-014-1131-8
- ↑ 16.0 16.1 Faden R (1992) Floral attraction and floral hairs in the Commelinaceae. Annals of the Missouri Botanical Garden 79(1): 46–52. https://doi.org/10.2307/2399808
- ↑ Fleming T, Estrada A (1993) Frugivory and seed dispersal: Ecological and evolutionary aspects. Kluwer Academic Publishers, Dordrecht, 392 pp. https://doi.org/10.1007/978-94-011-1749-4
- ↑ Thitimetharoch T (2004) Taxonomic studies of the family Commelinaceae in Thailand. PhD thesis, Khon Kaen University, India.
- ↑ Pellegrini M, Faden R, Almeida R (2016) Taxonomic revision of Neotropical Murdannia Royle (Commelinaceae). PhytoKeys 74: 35–78. https://doi.org/10.3897/phytokeys.74.9835
- ↑ Faden R (1978) Pollia Thunb. (Commelinaceae): The first generic record from the New World. Annals of the Missouri Botanical Garden 65(2): 676–680. https://doi.org/10.2307/2398866
- ↑ Faden R, Hunt D (1987) Reunion of Phaeosphaerion and Commelinopsis with Commelina (Commelinaceae). Annals of the Missouri Botanical Garden 74(1): 121–122. https://doi.org/10.2307/2399267

![Figure 4. Inflorescence architecture in Dichorisandrinae s.s. A diagram of the basic Siderasis Raf. emend M.Pell. & Faden inflorescence type, consisting of a thyrse reduced to a solitary cincinnus B diagram of the basic Dichorisandra inflorescence type (also characteristic of S. spectabilis and S. zorzonellii), consisting of a many-branched thyrse with alternate, many-flowered cincinni C diagram of the basic D. acaulis species group inflorescence type, where the main florescence axis and cincinni axis are greatly reduced, and the pedicels are peculiarly elongated. P = prophyll; pB = peduncle bract on main synflorescence axis; * = aborted or dormant apex of main inflorescence axis (usually not observed); B = cincinnus bract; b = bracteole; f = flower; 1°bud = bud terminating cincinnus; 2°bud = bud in axil of peduncle bract with potential to develop into a secondary thyrse (coflorescence); Modified from Pellegrini (2017)[9].](https://species-id.net/o/thumb.php?f=Phytokeys-83-001-g004.jpg&width=250)


