Milnesium matheusi
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Kaczmarek2019ZooKeys884, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Kaczmarek2019ZooKeys884">{{Citation See also the citation download page at the journal. |
Ordo: Apochela
Familia: Milnesiidae
Genus: Milnesium
Name
Milnesium matheusi Kaczmarek & Grobys & Kulpa & Bartylak & Kmita & Kepel & Kepel & Roszkowska, 2019 sp. nov. – Wikispecies link – ZooBank link – Pensoft Profile
Material examined
Holotype and 18 paratypes, all from sample No 139: Ivohibory forest, Madagascar, lichen sample from quartz rocks, coll. Marta Kepel and Andrzej Kepel.
Description
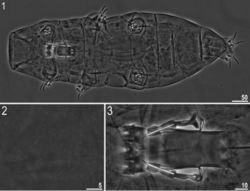
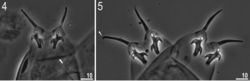
Adult females (Fig. 1, Table 4) with no modified claws I. Body light yellow before fixation and transparent afterwards, eyes present (in 89% of measured specimens). Dorsal cuticle sculptured with pseudopores, not arranged in bands, sparsely distributed and not forming a reticular design (Fig. 2). Six peribuccal papillae and six peribuccal lamellae present around the mouth opening. Two cephalic papillae positioned laterally. Peribuccal papillae slightly longer than lateral papillae.
The buccal apparatus of the Milnesium type (Figs 1, 3). The buccal tube wide and short (standard width, on average 46% of its length), and slightly funnel-shaped, wider anteriorly (posterior diameter on average 89% of the anterior diameter) (Table 4). The pharyngeal bulb elongated, pear-shaped and without placoids or septulum.
| Character | N | Range | Mean | SD | Holotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | µm | pt | ||||||
| Body length | 6 | 630 | – | 766 | – | – | – | 691 | – | 45 | – | 766 | – |
| Peribuccal papillae length | 5 | 10.0 | – | 12.0 | 18.6 | – | 22.1 | 11.0 | 19.9 | 0.8 | 1.5 | 11.8 | 18.9 |
| Lateral papillae length | 7 | 9.4 | – | 10.7 | 16.5 | – | 19.7 | 10.0 | 18.1 | 0.4 | 1.2 | 10.3 | 16.5 |
| Buccal tube | |||||||||||||
| Length | 9 | 51.3 | – | 62.5 | – | – | – | 56.6 | – | 3.8 | – | 62.5 | – |
| Stylet support insertion point | 9 | 34.5 | – | 42.3 | 66.1 | – | 69.4 | 38.4 | 67.8 | 2.4 | 1.3 | 41.5 | 66.4 |
| Anterior width | 9 | 25.2 | – | 35.9 | 47.6 | – | 57.9 | 28.9 | 51.0 | 3.2 | 3.1 | 31.4 | 50.2 |
| Standard width | 9 | 23.1 | – | 31.1 | 42.4 | – | 50.8 | 26.3 | 46.5 | 2.7 | 3.0 | 29.4 | 47.0 |
| Posterior width | 9 | 23.0 | – | 30.2 | 41.1 | – | 50.3 | 25.7 | 45.3 | 2.6 | 3.1 | 28.9 | 46.2 |
| Standard width/length ratio | 9 | 42% | – | 51% | – | – | – | 46% | – | 3% | – | 47% | – |
| Posterior/anterior width ratio | 9 | 84% | – | 94% | – | – | – | 89% | – | 4% | – | 92% | – |
| Claw 1 lengths | |||||||||||||
| External primary branch | 9 | 17.2 | – | 21.8 | 30.2 | – | 35.2 | 18.9 | 33.3 | 1.5 | 1.6 | 21.8 | 34.9 |
| External base + secondary branch | 9 | 13.3 | – | 16.7 | 23.5 | – | 27.9 | 15.0 | 26.5 | 1.2 | 1.5 | 16.6 | 26.6 |
| External spur | 7 | 3.5 | – | 5.3 | 6.4 | – | 9.6 | 4.4 | 7.8 | 0.7 | 1.3 | ? | ? |
| External branches length ratio | 9 | 76% | – | 82% | – | – | – | 80% | – | 2% | – | 76% | – |
| Internal primary branch | 9 | 16.0 | – | 21.1 | 30.2 | – | 34.5 | 18.3 | 32.3 | 1.6 | 1.6 | 21.1 | 33.8 |
| Internal base + secondary branch | 9 | 13.3 | – | 16.6 | 24.5 | – | 27.3 | 14.8 | 26.2 | 1.1 | 1.0 | 16.3 | 26.1 |
| Internal spur | 9 | 3.3 | – | 5.5 | 6.1 | – | 10.5 | 4.4 | 7.7 | 0.8 | 1.4 | 5.5 | 8.8 |
| Internal branches length ratio | 9 | 77% | – | 88% | – | – | – | 81% | – | 4% | – | 77% | – |
| Claw 2 lengths | |||||||||||||
| External primary branch | 8 | 17.4 | – | 21.2 | 32.9 | – | 36.5 | 19.5 | 34.9 | 1.4 | 1.4 | 21.2 | 33.9 |
| External base + secondary branch | 7 | 13.7 | – | 17.0 | 24.5 | – | 27.5 | 15.0 | 26.7 | 1.1 | 1.2 | 17.0 | 27.2 |
| External spur | 3 | 3.9 | – | 4.9 | 7.2 | – | 7.8 | 4.4 | 7.6 | 0.5 | 0.4 | 4.9 | 7.8 |
| External branches length ratio | 7 | 72% | – | 81% | – | – | – | 77% | – | 3% | – | 80% | – |
| Internal primary branch | 8 | 16.8 | – | 20.5 | 31.1 | – | 35.7 | 18.7 | 33.3 | 1.3 | 1.5 | 20.2 | 32.3 |
| Internal base + secondary branch | 9 | 13.0 | – | 16.3 | 25.0 | – | 27.9 | 14.7 | 26.0 | 1.1 | 0.9 | 16.3 | 26.1 |
| Internal spur | 9 | 3.4 | – | 5.8 | 6.1 | – | 10.3 | 4.4 | 7.8 | 0.8 | 1.5 | 4.7 | 7.5 |
| Internal branches length ratio | 8 | 74% | – | 81% | – | – | – | 78% | – | 3% | – | 81% | – |
| Claw 3 lengths | |||||||||||||
| External primary branch | 5 | 19.7 | – | 21.0 | 32.3 | – | 38.3 | 20.5 | 35.7 | 0.6 | 2.5 | ? | ? |
| External base + secondary branch | 6 | 14.2 | – | 16.3 | 24.5 | – | 28.4 | 15.4 | 27.1 | 0.7 | 1.5 | ? | ? |
| External spur | 5 | 3.5 | – | 5.2 | 6.4 | – | 9.3 | 4.4 | 7.7 | 0.7 | 1.1 | ? | ? |
| External branches length ratio | 5 | 72% | – | 82% | – | – | – | 75% | – | 4% | – | ? | – |
| Internal primary branch | 5 | 18.9 | – | 20.4 | 31.3 | – | 36.5 | 19.7 | 34.3 | 0.6 | 2.0 | ? | ? |
| Internal base + secondary branch | 6 | 13.7 | – | 16.0 | 23.7 | – | 28.2 | 14.9 | 26.2 | 0.8 | 1.9 | ? | ? |
| Internal spur | 5 | 3.8 | – | 5.6 | 7.0 | – | 9.7 | 4.8 | 8.3 | 0.7 | 1.1 | ? | ? |
| Internal branches length ratio | 5 | 70% | – | 79% | – | – | – | 75% | – | 4% | – | ? | – |
| Claw 4 lengths | |||||||||||||
| Anterior primary branch | 7 | 19.6 | – | 23.0 | 35.1 | – | 39.8 | 20.9 | 37.2 | 1.3 | 1.5 | 23.0 | 36.8 |
| Anterior base + secondary branch | 7 | 14.6 | – | 17.2 | 26.3 | – | 29.4 | 15.8 | 28.2 | 0.9 | 1.1 | 17.2 | 27.5 |
| Anterior spur | 6 | 4.1 | – | 6.3 | 7.5 | – | 11.5 | 5.4 | 9.7 | 0.9 | 1.6 | 6.0 | 9.6 |
| Anterior branches length ratio | 7 | 71% | – | 80% | – | – | – | 76% | – | 4% | – | 75% | – |
| Posterior primary branch | 7 | 20.5 | – | 24.0 | 38.1 | – | 41.3 | 21.8 | 38.9 | 1.1 | 1.1 | 24.0 | 38.4 |
| Posterior base + secondary branch | 7 | 15.2 | – | 17.7 | 26.9 | – | 29.6 | 16.1 | 28.6 | 0.8 | 1.0 | 17.7 | 28.3 |
| Posterior spur | 7 | 4.4 | – | 5.8 | 7.6 | – | 10.3 | 5.2 | 9.3 | 0.6 | 1.1 | 5.5 | 8.8 |
| Posterior branches length ratio | 7 | 70% | – | 76% | – | – | – | 74% | – | 2% | – | 74% | – |
Eggs oval, smooth and deposited in the exuvium as in all other known Milnesium species.
| Character | N | Range | Mean | SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | ||||||
| Body length | 2 | 409 | – | 428 | – | – | – | 419 | – | 13 | – |
| Peribuccal papillae length | 3 | 3.0 | – | 3.9 | 8.9 | – | 11.3 | 3.5 | 10.2 | 0.5 | 1.2 |
| Lateral papillae length | 3 | 5.6 | – | 6.0 | 16.2 | – | 17.8 | 5.9 | 17.1 | 0.2 | 0.8 |
| Buccal tube | |||||||||||
| Length | 3 | 33.8 | – | 34.5 | – | – | – | 34.2 | – | 0.4 | – |
| Stylet support insertion point | 2 | 21.2 | – | 22.3 | 62.7 | – | 64.6 | 21.8 | 63.7 | 0.8 | 1.4 |
| Anterior width | 3 | 9.4 | – | 11.2 | 27.8 | – | 32.6 | 10.5 | 30.8 | 1.0 | 2.6 |
| Standard width | 3 | 9.1 | – | 9.8 | 26.9 | – | 28.5 | 9.5 | 27.8 | 0.4 | 0.8 |
| Posterior width | 3 | 9.4 | – | 10.2 | 27.8 | – | 29.6 | 9.8 | 28.7 | 0.4 | 0.9 |
| Standard width/length ratio | 3 | 27% | – | 28% | – | – | – | 28% | – | 1% | – |
| Posterior/anterior width ratio | 3 | 88% | – | 100% | – | – | – | 94% | – | 6% | – |
| Claw 1 lengths | |||||||||||
| External primary branch | 2 | 15.8 | – | 16.3 | 45.9 | – | 48.2 | 16.1 | 47.1 | 0.4 | 1.6 |
| External base + secondary branch | 3 | 14.1 | – | 15.0 | 41.7 | – | 43.5 | 14.6 | 42.7 | 0.5 | 0.9 |
| External spur | 2 | 3.2 | – | 3.4 | 9.3 | – | 9.9 | 3.3 | 9.6 | 0.1 | 0.4 |
| External branches length ratio | 2 | 87% | – | 94% | – | – | – | 90% | – | 5% | – |
| Internal primary branch | 3 | 14.9 | – | 15.7 | 43.2 | – | 46.4 | 15.4 | 45.1 | 0.5 | 1.7 |
| Internal base + secondary branch | 3 | 14.0 | – | 14.5 | 40.6 | – | 42.9 | 14.2 | 41.4 | 0.3 | 1.3 |
| Internal spur | 3 | 3.0 | – | 3.7 | 8.9 | – | 10.7 | 3.4 | 9.9 | 0.4 | 0.9 |
| Internal branches length ratio | 3 | 89% | – | 94% | – | – | – | 92% | – | 2% | – |
| Claw 2 lengths | |||||||||||
| External primary branch | 2 | 16.9 | – | 17.9 | 49.0 | – | 53.0 | 17.4 | 51.0 | 0.7 | 2.8 |
| External base + secondary branch | 1 | 13.2 | – | 13.2 | 39.1 | – | 39.1 | 13.2 | 39.1 | ? | ? |
| External spur | 1 | 3.5 | – | 3.5 | 10.4 | – | 10.4 | 3.5 | 10.4 | ? | ? |
| External branches length ratio | 1 | 74% | – | 74% | – | – | – | 74% | – | ? | – |
| Internal primary branch | 3 | 16.4 | – | 16.9 | 47.5 | – | 50.0 | 16.7 | 48.8 | 0.3 | 1.2 |
| Internal base + secondary branch | 2 | 12.7 | – | 12.8 | 37.2 | – | 37.6 | 12.8 | 37.4 | 0.1 | 0.3 |
| Internal spur | 2 | 3.5 | – | 5.0 | 10.4 | – | 14.5 | 4.3 | 12.4 | 1.1 | 3.0 |
| Internal branches length ratio | 2 | 75% | – | 76% | – | – | – | 76% | – | 1% | – |
| Claw 3 lengths | |||||||||||
| External primary branch | 3 | 16.2 | – | 17.4 | 47.1 | – | 51.5 | 16.8 | 49.0 | 0.6 | 2.3 |
| External base + secondary branch | 2 | 12.1 | – | 12.8 | 35.2 | – | 37.9 | 12.5 | 36.5 | 0.5 | 1.9 |
| External spur | 1 | 3.9 | – | 3.9 | 11.3 | – | 11.3 | 3.9 | 11.3 | ? | ? |
| External branches length ratio | 2 | 74% | – | 75% | – | – | – | 74% | – | 1% | – |
| Internal primary branch | 3 | 14.8 | – | 17.0 | 43.0 | – | 50.3 | 16.0 | 46.7 | 1.1 | 3.6 |
| Internal base + secondary branch | 2 | 12.7 | – | 13.0 | 37.6 | – | 37.8 | 12.9 | 37.7 | 0.2 | 0.2 |
| Internal spur | 2 | 2.9 | – | 4.0 | 8.4 | – | 11.8 | 3.5 | 10.1 | 0.8 | 2.4 |
| Internal branches length ratio | 2 | 75% | – | 88% | – | – | – | 81% | – | 9% | – |
| Claw 4 lengths | |||||||||||
| Anterior primary branch | 3 | 16.3 | – | 17.0 | 47.4 | – | 49.3 | 16.6 | 48.5 | 0.4 | 1.0 |
| Anterior base + secondary branch | 2 | 12.4 | – | 12.9 | 36.7 | – | 37.5 | 12.7 | 37.1 | 0.4 | 0.6 |
| Anterior spur | 1 | 3.8 | – | 3.8 | 11.0 | – | 11.0 | 3.8 | 11.0 | ? | ? |
| Anterior branches length ratio | 2 | 75% | – | 79% | – | – | – | 77% | – | 3% | – |
| Posterior primary branch | 3 | 17.7 | – | 18.8 | 51.3 | – | 54.7 | 18.3 | 53.4 | 0.6 | 1.8 |
| Posterior base + secondary branch | 3 | 12.7 | – | 13.7 | 37.1 | – | 39.8 | 13.1 | 38.2 | 0.6 | 1.5 |
| Posterior spur | 2 | 3.0 | – | 4.1 | 8.7 | – | 11.9 | 3.6 | 10.3 | 0.8 | 2.3 |
| Posterior branches length ratio | 3 | 69% | – | 73% | – | – | – | 72% | – | 2% | – |
DNA sequences
We obtained good quality sequences for the applied molecular markers: 28S rRNA sequence (GenBank: MN191503), 756 bp long; COI sequence (GenBank: MN187056), 628 bp long; ITS-2 sequence (GenBank: MN239906), 218 bp long.
Type locality
Madagascar, 22°37'07.7"S, 46°43'14.5"E, ca. 1187 m asl, Fianarantsoa Province, Ivohibory forest.
Etymology
The second author with great pleasure dedicates this species to her fiance – Mateusz Wojciechowski.
Type depositories
The holotype and 13 paratypes (slides: MAD139/14, MAD139/16, MAD139/18, MAD139/19, MAD139/34, MAD139/35, MAD139/42, MAD139/56, MAD139/72) are deposited at the Department of Animal Taxonomy and Ecology, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 6, Poznań, Poland; five paratypes (slides: MAD139/12, MAD139/13, MAD139/15) are deposited at the Natural History Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark.
Morphological differential diagnosis
The new species with three points on the secondary branches of all claws (claw configuration [3-3]–[3-3]) and a rather wide buccal tube, in relation to its length, is most similar to: Mil. beatae Roszkowska, Ostrowska & Kaczmarek, 2015, Mil. bohleberi Bartels, Nelson, Kaczmarek & Michalczyk, 2014, Mil. eurystomum Maucci, 1991, Mil. shilohae Meyer, 2015 and Mil. tumanovi Pilato, Sabella & Lisi, 2016, but it differs from:
1. Milnesium beatae, only reported from Argentina and USA (Roszkowska et al. 2015[1]; Tibbs et al. 2016[2]) by: narrower buccal tube (25.2–35.9 [47.6–57.9] and 23.1–31.1 [42.4–50.8] anterior and standard width, respectively, in the new species vs. 37.0–53.5 [70.3–78.9] and 32.0–42.5 [58.1–65.6] anterior and standard width respectively in Mil. beatae), smaller standard width/length ratio of the buccal tube (42%–51% in new species vs. 58%–66% in Mil. beatae) and larger posterior/anterior width ratio (84%–94% in new species vs. 69%–76% in Mil. beatae).
2. Milnesium bohleberi, only recorded from North Carolina and Tennessee (USA) (Bartels et al. 2014[3]) by: presence of pseudopores on dorsal cuticle, shorter peribuccal papillae (10.0–12.0 [18.6–22.1] in new species vs. 15.5–20.3 [27.2–32.3] in Mil. bohleberi), smaller pt values of anterior, standard and posterior widths of the buccal tube (47.6–57.9, 42.4–50.8, 41.1–50.3, respectively, in new species vs. 63.4–74.7, 54.5–64.0, 52.4–62.0, respectively, in Mil. bohleberi), smaller standard width/length ratio of the buccal tube (42%–51% in new species vs. 54%–64% in Mil. bohleberi) and slightly shorter claws (see Table 4 below and Bartels et al. (2014[3]: Table 1) for the exact differences in claw dimensions).
3. Milnesium eurystomum reported from a few localities in Argentina, Chile, Greenland, Mongolia and USA (see review by Kaczmarek et al. 2016[4]) by: shorter buccal tube (51.3–62.5 in new species vs. 70.8–77.5 in Mil. eurystomum), stylet supports inserted in a more posterior position (pt = 66.1–69.4 in new species vs. ca. pt = 60.0–60.3 in Mil. eurystomum), narrower buccal tube (25.2–35.9 [47.6–57.9], 23.1–31.1 [42.4–50.8] and 23.0–30.2 [41.1–50.3] anterior, standard and posterior width, respectively, in new species vs. 53.7–55.9 [72.1–75.8], 45.9–47.9 [61.8–64.8] and 33.9–41.0 [43.7–57.9] anterior, standard and posterior width, respectively, in Mil. eurystomum), smaller standard width/length ratio of the buccal tube (42%–51% in new species vs. 62%–65% in Mil. eurystomum) and larger posterior/anterior width ratio (84%–94% in new species vs. 61%–76% in Mil. eurystomum).
4. Milnesium shilohae, only reported from the type locality in Hawaii (USA) (Meyer 2015[5]) by: presence of pseudopores on dorsal cuticle, presence of similar in length spurs on internal and external claws (internal and posterior spurs larger than external and anterior spurs in Mil. shilohae), slightly longer lateral papillae (9.4–10.7 in new species vs. 5.0–9.0 in Mil. shilohae), slightly longer buccal tube (51.3–62.5 in new species vs. 38.4–50.3 in Mil. shilohae), stylet supports inserted in a more anterior position (pt = 66.1–69.4 in new species vs. pt = 75.5–77.5 in Mil. shilohae) and larger spurs on some external and anterior claws (see Table 4 below and Table 3 in Meyer (2015)[5] for the exact differences in claw dimensions).
5. Milnesium tumanovi, only recorded from the type locality in the Crimea (Ukraine) (Pilato et al. 2016[6]) by: presence of pseudopores on dorsal cuticle, funnel-shaped buccal tube (cylindrical in Mil. tumanovi) and stylet supports inserted in a more posterior position (pt = 66.1–69.4 in new species vs. ca. pt = 52–54 in Mil. tumanovi).
Genotypic differential diagnosis
The ranges of uncorrected genetic p-distances between Mil. matheusi sp. nov. and species of the genus Milnesium, for which molecular marker sequences are available from GenBank (see Table 6 for details), are as follows:
| DNA marker | Taxon | Accession number | Source |
|---|---|---|---|
| 28S rRNA | Milnesium sp. | JX888585.1 | Adams et. al. unpublished |
| JX888586.1 | Adams et. al. unpublished | ||
| JX888587.1 | Adams et. al. unpublished | ||
| Milnesium tardigradum | JX888541.1 | Adams et. al. unpublished | |
| JX888540.1 | Adams et. al. unpublished | ||
| KC138808.1 | Zawierucha unpublished | ||
| KC138809.1 | Zawierucha unpublished | ||
| Milnesium sp. | AY210826.1 | Mallatt et. al. 2004[7] | |
| Milnesium tardigradum | FJ435780.1 | Guil and Giribet 2012[8] | |
| FJ435779.1 | Guil and Giribet 2012[8] | ||
| Milnesium berladnicorum | KT951661.1 | Morek et. al. 2016[9] | |
| Milnesium variefidum | KT951665.1 | Morek et. al. 2016[9] | |
| COI | Milnesium sp. | KX306950.1 | Fox et al. unpublished |
| Milnesium tardigradum | EU244603.1 | Schill unpublished | |
| EU244604 | Schill unpublished | ||
| FJ435810.1 | Guil and Giribet 2012[8] | ||
| Milnesium t. tardigradum | JN664950.1 | Michalczyk et al. 2012[10] | |
| Milnesium cf. tardigradum | JX683824.1 | Vicente et al. 2013[11] | |
| JX683823.1 | Vicente et al. 2013[11] | ||
| JX683822.1 | Vicente et al. 2013[11] | ||
| Milnesium sp. | KJ857002.1 | Velasco-Castrillón et al. 2015[12] | |
| KJ857001.1 | Velasco-Castrillón et al. 2015[12] | ||
| Milnesium cf. alpigenum | KU513422.1 | Kosztyła et al. 2016[13] | |
| Milnesium variefidum | KT951663.1 | Morek et al. 2016[9] | |
| Milnesium berladnicorum | KT951659.1 | Morek et al. 2016[9] | |
| Milnesium sp. | EF632553.1 | Sands et. al unpublished | |
| Milnesium cf. granulatum | MH751517.1 | Jackson and Meyer 2019[14] | |
| Milnesium lagniappe | MH751518.1 | Jackson and Meyer 2019[14] | |
| Milnesium tardigradum | MG923558.1 | Morek et al. 2019[15] | |
| MG923559.1 | Morek et al. 2019[15] | ||
| MG923560.1 | Morek et al. 2019[15] | ||
| MG923561.1 | Morek et al. 2019[15] | ||
| MG923562.1 | Morek et al. 2019[15] | ||
| MG923563.1 | Morek et al. 2019[15] | ||
| MG923564.1 | Morek et al. 2019[15] | ||
| MG923565.1 | Morek et al. 2019[15] | ||
| Milnesium dornensis | MG923566.1 | Morek et al. 2019[15] | |
| ITS-2 | Milnesium alpigenum | MH000382.1 | Morek et al. unpublished |
| Milnesium sp. | MH000386.1 | Morek et al. unpublished | |
| MH000387.1 | Morek et al. unpublished | ||
| Milnesium tardigradum | HM150648.2 | Wełnicz et. al. 2010[16] | |
| GQ403682.1 | Schill et al. 2010[17] | ||
| GQ403681.1 | Schill et al. 2010[17] | ||
| Milnesium t. tardigradum | JF951049 | Michalczyk et al. 2012[10] | |
| Milnesium variefidum | KT951667.1 | Morek et. al. 2016[9] | |
| KT951666.1 | Morek et. al. 2016[9] | ||
| Milnesium berladnicorum | KT951662.1 | Morek et. al. 2016[9] | |
| Milnesium cf. granulatum | MK681875.1 | Jackson and Meyer 2019[14] | |
| MK681876.1 | Jackson and Meyer 2019[14] | ||
| MK681877.1 | Jackson and Meyer 2019[14] | ||
| MK681878.1 | Jackson and Meyer 2019[14] | ||
| MK681879.1 | Jackson and Meyer 2019[14] | ||
| MK681880.1 | Jackson and Meyer 2019[14] | ||
| MK681881.1 | Jackson and Meyer 2019[14] | ||
| MK681882.1 | Jackson and Meyer 2019[14] | ||
| MK681883.1 | Jackson and Meyer 2019[14] | ||
| MK681884.1 | Jackson and Meyer 2019[14] | ||
| MK681885.1 | Jackson and Meyer 2019[14] | ||
| MK681886.1 | Jackson and Meyer 2019[14] |
2. COI: 20.1–38.8% (23.3% on average), with the most similar being Mil. variefidum Morek, Gąsiorek, Stec, Blagden & Michalczyk, 2016 from UK (KT951663.1) (Morek et al. 2016[9]) and the least similar being Mil. t. tardigradum from Spain (FJ435810.1) (Guil and Giribet 2012[8]);
3. ITS-2: 17.8–31.1% (23.7% on average), with the most similar being Mil. t. tardigradum from Germany (JF951049.1) (Michalczyk et al. 2012[10]) and the least similar being Mil. cf. granulatum from USA (MK681879.1) (Jackson and Meyer 2019[14]).
Original Description
- Kaczmarek, Ł; Grobys, D; Kulpa, A; Bartylak, T; Kmita, H; Kepel, M; Kepel, A; Roszkowska, M; 2019: Two new species of the genus Milnesium Doyère, 1840 (Tardigrada, Apochela, Milnesiidae) from Madagascar ZooKeys, 884: 1-22. doi
Images
|
Other References
- ↑ Roszkowska M, Ostrowska M, Kaczmarek Ł (2015) The genus Milnesium Doyère, 1840 (Tardigrada) in South America with descriptions of two new species from Argentina and discussion of the feeding behaviour in the family Milnesiidae.Zoological Studies54(1): 1–12. https://doi.org/10.1186/s40555-014-0082-7
- ↑ Tibbs L, Emanuels A, Miller W (2016) Tardigrades of the canopy: Argentine species Milnesium beatae Roszkowska, Ostrowska and Kaczmarek, 2015 (Eutardigrada, Milnesidae) discovered in the trees of Kansas, USA.Transactions of the Kansas Academy of Science119(2): 173–178. https://doi.org/10.1660/062.119.0207
- ↑ 3.0 3.1 Bartels P, Nelson D, Kaczmarek Ł, Michalczyk Ł (2014) The genus Milnesium (Tardigrada: Eutardigrada: Milnesiidae) in the Great Smoky Mountains National Park (North Carolina and Tennessee, USA), with the description of Milnesium bohleberi sp. nov.Zootaxa3826(2): 356–368. https://doi.org/10.11646/zootaxa.3826.2.5
- ↑ Kaczmarek Ł, Michalczyk Ł, Sandra J (2016) Annotated zoogeography of non-marine Tardigrada. Part III: North America and Greenland.Zootaxa4203(1): 1–249. https://doi.org/10.11646/zootaxa.4203.1.1
- ↑ 5.0 5.1 Meyer H (2015) Water bears (Phylum Tardigrada) of Oceania, with the description of a new species of Milnesium.New Zealand Journal of Zoology42(3): 173–186. https://doi.org/10.1080/03014223.2015.1062402
- ↑ Pilato G, Sabella G, Lisi O (2016) Two new species of Milnesium (Tardigrada: Milnesiidae).Zootaxa4132(4): 575–587. https://doi.org/10.11646/zootaxa.4132.4.9
- ↑ Mallatt J, Garey J, Shultz J (2004) Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin.Molecular Phylogenetic and Evolution31(1): 178–191. https://doi.org/10.1016/j.ympev.2003.07.013
- ↑ 8.0 8.1 8.2 8.3 Guil N, Giribet G (2012) A comprehensive molecular phylogeny of tardigrades adding genes and taxa to a poorly resolved phylum-level phylogeny.Cladistics28(1): 21–49. https://doi.org/10.1111/j.1096-0031.2011.00364.x
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Morek W, Gąsiorek P, Stec D, Blagden B, Michalczyk Ł (2016) Experimental taxonomy exposes ontogenetic variability and elucidates the taxonomic value of claw configuration in Milnesium Doyère, 1840 (Tardigrada: Eutardigrada: Apochela).Contributions to Zoology85(2): 173–200. https://doi.org/10.1163/18759866-08502003
- ↑ 10.0 10.1 10.2 Michalczyk Ł, Wełnicz W, Frohme M, Kaczmarek Ł (2012) Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus.Zootaxa3154: 1–20. https://doi.org/10.11646/zootaxa.3154.1.1
- ↑ 11.0 11.1 11.2 Vicente F, Cesari M, Serrano A, Bertolani R (2013) The impact of fire on terrestrial tardigrade biodiversity: a first case-study from Portugal. Journal of Limnology 72(S1): 152–159. https://doi.org/10.4081/jlimnol.2013.s1.e19
- ↑ 12.0 12.1 Velasco-Castrillón A, McInnes S, Schultz M, Arróniz-Crespo M, D’Haese C, Gibson J, Adams B, Page T, Austin A, Cooper S, Stevens M (2015) Mitochondrial DNA analyses reveal widespread tardigrade diversity in Antarctica.Invertebrate Systematics29: 578–590. https://doi.org/10.1071/IS14019
- ↑ Kosztyła P, Stec D, Morek W, Gąsiorek P, Zawierucha K, Michno K, Ufir K, Małek D, Hlebowicz K, Laska A, Dudziak M, Frohme M, Prokop Z, Kaczmarek Ł, Michalczyk Ł (2016) Experimental taxonomy confirms the environmental stability of morphometric traits in a taxonomically challenging group of microinvertebrates.Zoological Journal of the Linnaean Society178(4): 765–775. https://doi.org/10.1111/zoj.12409
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 14.11 14.12 14.13 14.14 Jackson K, Meyer H (2019) Morphological and genetic analysis of Milnesium cf. granulatum (Tardigrada: Milnesiidae) from Northeastern North America.Zootaxa4604(3): 497–510. https://doi.org/10.11646/zootaxa.4604.3.6
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 15.8 Morek W, Stec D, Gąsiorek P, Surmacz B, Michalczyk Ł (2019) Milnesium tardigradum Doyère, 1840: The first integrative study of interpopulation variability in a tardigrade species.Journal of Zoological Systematics and Evolutionary Research57: 1–23. https://doi.org/10.1111/jzs.12233
- ↑ Wełnicz W, Grohme M, Kaczmarek Ł, Schill R, Frohme M (2010) ITS-2 and 18S rRNA data from Macrobiotus polonicus and Milnesium tardigradum (Eutardigrada, Tardigrada). Journal of Zoological Systematic and Evolutionary Research 49(Suppl. 1): 34–39. https://doi.org/10.1111/j.1439-0469.2010.00595.x
- ↑ 17.0 17.1 Schill R, Forster F, Dandekar T, Wolf M (2010) Using compensatory base change analysis of internal transcribed spacer 2 secondary structures to identify three new species in Paramacrobiotus (Tardigrada).Organism Diversity and Evolution10(4): 287–296. https://doi.org/10.1007/s13127-010-0025-z