Microporella ichnusae
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Martino2021ZooKeys1053, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Martino2021ZooKeys1053">{{Citation See also the citation download page at the journal. |
Ordo: Cheilostomatida
Familia: Microporellidae
Genus: Microporella
Name
Microporella ichnusae Martino & Rosso, 2021 sp. nov. – Wikispecies link – ZooBank link – Pensoft Profile
- Microporella sp. A Fraschetti et al. 2010[1]: table 27.
Type material
Holotype: Italy • 1 living colony consisting of more than 100 zooids, including some complete and some broken ovicells; Iberian-Provençal Basin, NW Sardinia, Capo Caccia–Isola Piana MPA, Bisbe submarine cave; sample Bisbe 2; 40°34'15"N, 8°12'55"E; 8 m; 2009; V. Di Martino leg.; scuba diving; GSO Biocoenosis; PMC. B30a. 20.11.2020. Paratypes: Italy • 9 living colonies, each consisting of a dozen zooids; Iberian-Provençal Basin, NW Sardinia, Capo Caccia–Isola Piana MPA, Bisbe, Falco and Galatea caves; samples Bisbe 1, Bisbe 2, Falco 2, Galatea 1 and Galatea 2; Bisbe, same details as the holotype; Falco: 40°34'09"N, 8°13'14"E; Galatea: 40°34'09"N, 8°13'54"E; 4–8 m; 2008; V. Di Martino leg.; scuba diving; GSO Biocoenosis; PMC. B30b. 20.11.2020.
Diagnosis
Colony encrusting, multiserial. Autozooid frontal shield densely pustulose and sparsely pseudoporous. Orifice transversely D-shaped; hinge-line smooth with blunt condyles close to corners; four thin oral spines, hidden in ovicellate zooids. Ascopore field semi-elliptical; ascopore opening an arched fissure marked by a distal tongue with radial spines. Avicularium usually single, same level as or proximal to the ascopore, occasionally paired, directed distolaterally; crossbar complete; rostrum lanceolate, channelled. Ovicell non-personate.
Description
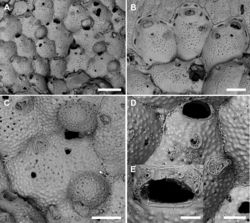
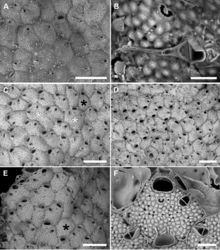
Colony encrusting multiserial, unilaminar (Fig. 5C, D) with zooids arranged in alternate rows often disrupted on particularly irregular substrata; interzooidal communications through 6–8 elliptical pore chamber windows (40–65 × 18–26 µm).
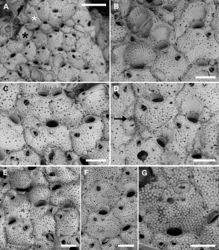
Autozooids usually hexagonal to rhomboidal but sometimes irregularly shaped, 307–587 (434±73, N = 20) × 284–439 (357±59, N = 20 µm) (mean L/W = 1.21), boundaries marked by narrow grooves and raised rims of lateral walls (Figs 5F, 6B, G). Frontal shield slightly convex with polygonal and flat-topped pustules giving a tessellate appearance, and pierced by circular (diameter 6–12 µm), irregularly distributed pseudopores, usually numbering 10–30 but more numerous in some colonies; 2–4 marginal areolae, elliptical to fissure-like, barely visible or distinguishable from pseudopores (Figs 5F, 6B). Orifice transversely D-shaped, 75–94 (81±5, N = 20) × 109–145 (122±10, N = 20) µm (mean OL/OW = 0.67; mean ZL/OL = 5.33), outlined by a thin, slightly raised rim; hinge-line straight, smooth, with a pair of small triangular, blunt condyles close to corners (Fig. 5F). Oral spines four, occasionally five or six (diameter of the bases 10–14 μm), evenly spaced, the proximal pair located at orifice mid-length; spines hidden in ovicellate autozooids (Fig. 6B, E).
Ascopore field a small and very narrow, transversely semi-elliptical area marked by a thin raised gymnocystal rim, 28–36 × 30–50 μm, located 25–50 μm below the orifice, at the same level as the frontal shield; opening transversely C-shaped, 20–30 × 6–10 μm, with a subcircular tongue projecting from distal edge, and relatively few, tiny, radial denticles.
Avicularium most often single (Figs 5E, F, 6B, D), occasionally paired (Fig. 5C) or absent (Figs 5C, E, 6C), relatively large, 75–120 (98±13, N = 20) × 55–91 (71±11, N = 20) μm (mean AvL/AvW = 1.39), located laterally, on either side, in the distal zooidal half, same level as or proximally to the ascopore (Figs 5B, F, 6B, E) but on irregularly shaped zooids occasionally placed in the proximal part; crossbar complete, thin; rostrum triangular, channelled and open-ended, directed laterally or distolaterally, often distally raised on a smooth, gymnocystal cystid (Fig. 5F). Mandible 160–180 μm long, setiform, with a hook at about one-third of its length that clamps it to the rostrum tip, crossing the whole zooid when open (Fig. 5A, B). Ovicell subglobular and prominent, 185–241 (214±25, N = 4) × 290–314 (297±11, N = 4) μm (mean OvL/OvW = 0.72), produced by and continuous with frontal shield of distal zooid, obscuring distal part of the orifice; calcification fabric similar to frontal shield but with smaller and more prominent pustules; pseudopores small (diameter 5–10 μm), densely packed at the periphery, absent centrally (Fig. 6B, E).
Kenozooids smaller than or nearly as large as autozooids, lacking openings such as orifices and ascopores but sometimes equipped with avicularium (Fig. 6A, F).
Ancestrula not observed.
Etymology
From Ichnusa the Latinized form of the ancient Greek name for Sardinia.
Remarks
Size and shape of autozooids vary remarkably within and between colonies, including dwarf-like autozooids, about half the size of the more regular ones, as well as extremely large and irregularly shaped autozooids, appearing as the result of the fusion of contiguous autozooids (Fig. 5C, E). In this latter case the avicularium can be placed much more proximally than in regular autozooids. Irregularly-shaped autozooids also occur in M. browni (Harmelin et al. 2011[2]: fig. 3b). These unusual autozooids and the kenozooids observed in this species seem to be particularly common in zones of contact between colonies or lobes of the same colony, and in damaged areas, also associated with evidences of reparation (Figs 5D, 6A, D), such as regeneration of broken autozooids, patches of calcification to close holes in the frontal shield, or orifices of presumably not functional autozooids (Fig. 6G). Intrazooidal budding, a feature that is common in bryozoans from submarine caves (e.g., Rosso et al. 2020a[3], 2020b[4]), has been more commonly observed in avicularia (Fig. 6C, D) than autozooids (Fig. 6F, G). The occurrence of ovicells seems rare, observed only on the colony selected as the holotype.
The general appearance of this new species is very similar to M. ciliata. However, the orifice in M. ciliata, although of comparable size (0.06–0.08 mm long by 0.11–0.15 mm wide), is proportionately shorter, the hinge-line shows a series of median denticles and the two lateral condyles are more prominent and more laterally placed (Fig. 4E; see also Kukliński and Taylor 2008[5]: fig. 1G). The type and position of the oral spines are similar but the number of spines is 4–6 (more commonly four) in M. ichnusae sp. nov. and 1–4 (and occasionally lacking in the zone of astogenetic repetition) in M. ciliata (Kukliński and Taylor 2008[5]). In M. ciliata the frontal avicularium is constantly single, only lacking in the first autozooid budded from the ancestrula, and no kenozooids were reported (Kukliński and Taylor 2008[5]). Furthermore, the ovicells in M. ciliata have length comparable with those of M. ichnusae sp. nov. but are much narrower.
Distribution and ecology
Microporella ichnusae sp. nov. is presently known only from submarine caves in the Capo Caccia-Isola Piana MPA, in NW Sardinia. However, it is possible that some previous records of M. ciliata, to date the only Microporella species with a single avicularium considered as widespread in the Mediterranean, belong to this species.
Original Description
- Martino, E; Rosso, A; 2021: Seek and ye shall find: new species and new records of Microporella (Bryozoa, Cheilostomatida) in the Mediterranean ZooKeys, 1053: 1-42. doi
Images
|
Other References
- ↑ Fraschetti S, Boero F, Guarnieri G, Terlizzi A, Guidetti P, Bussotti S, Piraino S, De Vito D, Cormaci M, Furnari G, Catra M, Alongi G, Rosso A, Di Martino E, Ceccherelli G, Manconi R, Ledda F, Cattaneo-Vietti R, Cerrano C, Pantaleo U, Scinto A, Bavestrello G, Di Camillo C, Betti F, Chemello R, Milazzo M, Graziano M, Di Franco A, Marchini A, Russo G, Di Stefano F, Cimmino P (2010) Studio degli ambienti di grotte marine sommerse (Cod.8330) nelle aree marine protette di Capo Caccia, Plemmirio e Isole Pelagie. Relazione finale. CoNISMa-Ministero dell’Ambiente e della Tutela del Territorio e del Mare, 337 pp.
- ↑ Harmelin J, Ostrovsky A, Cáceres-Chamizo J, Sanner J (2011) Bryodiversity in the tropics: taxonomy of Microporella species (Bryozoa, Cheilostomata) with personate maternal zooids from Indian Ocean, Red Sea and southeast Mediterranean.Zootaxa2798: 1–30. https://doi.org/10.11646/zootaxa.2798.1.1
- ↑ Rosso A, Di Martino E, Gerovasileiou V (2020a) Revision of the genus Setosella (Bryozoa: Cheilostomata) with description of new species from deep-waters and submarine caves of the Mediterranean.Zootaxa4728(4): 401–442. https://doi.org/10.11646/zootaxa.4728.4.1
- ↑ Rosso A, Gerovasileiou V, Di Martino E (2020b) Really onychocellids? Revisions and new findings increase the astonishing bryozoan diversity of the Mediterranean Sea. In: Crocetta F (Ed.) Benthic Biodiversity in the Northeastern Atlantic and the Mediterranean Sea, Journal of Marine Science and Engineering, sec. Marine Biology 8(11): e904. https://doi.org/10.3390/jmse8110904
- ↑ 5.0 5.1 5.2 Kukliński P, Taylor P (2008) Arctic species of the cheilostome bryozoan Microporella, with a redescription of the type species.Journal of Natural History42(27–28): 1893–1906. https://doi.org/10.1080/00222930802126904