Longitarsus limnophilae
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Prathapan2011ZooKeys87, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Prathapan2011ZooKeys87">{{Citation See also the citation download page at the journal. |
Ordo: Coleoptera
Familia: Chrysomelidae
Genus: Longitarsus
Name
Longitarsus limnophilae Prathapan & Viraktamath sp. n. – Wikispecies link – ZooBank link – Pensoft Profile
Holotype
♂, with labels as follows: “INDIA Kerala / Vembayam / 12. ix. 2009 Prathapan Coll.” “Longitarsus limnophilae sp. nov. / Prathapan & Viraktamath” “HOLOTYPE [red printed label]” (BMNH).
Paratypes
(30 specimens): 7 ♂, 3 ♀. The same labels as holotype; 5 ♀. same data as for holotype except dating 3.x.2009; 5 ♂. same data except dating 24.x.2009; 9 ♂, 1 ♀. same data except dating 16.i.2010 (5 BMNH, 5 USNM, 5 UASB, 12 NPC, 3 PKDC).
Etymology
This unique species is named after its host plant. The name is a noun in the genitive case.
Description
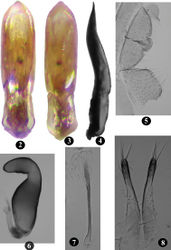
Length 1.89 – 2.15 mm; width 0.91 – 1.08 mm; female (2.09 – 2.15 mm) slightly larger than male (1.89 – 2.12 mm). General color brown (Fig. 1). Fore- and middle legs, hind tibia and tarsi light brown. Antenna piceous with proximal three to five antennomeres gradually turning brown. Labrum dark brown to piceous, suture narrowly piceous. Ventrites lighter than dorsum.
Vertex shiny, impunctate, minutely wrinkled. Ommatidia fully developed. Postcallinal sulcus weak but distinct. Frontal ridge unusual, broad and not sharply raised, anteriorly widening towards frontoclypeal suture, anteriorly forming ill-defined denticle in middle of flat, poorly developed anterofrontal ridge. Maxillary palpus with last palpomere longest. Antenna extends well beyond apex of elytra over pronotum. Second antennomere longer than half of third; second and third together longer than first, subequl to fourth; fifth longer than fourth; fifth to seventh subequal, eighth to tenth progressively shorter than previous antennomere. Pronotum anteriorly wider than posteriorly; 1.27 – 1.34 times wider than long; anterolateral callosity posteriorly lower than anteriorly, not forming denticle at pore; posterolateral callosity protrudes beyond lateral margin; lateral margin weakly curved, anteriorly broader than posteriorly; disc shiny with minute punctures more evident posteriorly. Elytra with well developed humeral calli, punctures distinct, width of interstices smaller than diameter of one puncture in middle of elytron. Elytral apex convex, with one long seta. Hind wings well developed. Scutellum triangular. First male protarsomere 1.60 – 1.67 times longer than wide; first female protarsomere 2.00 – 2.43 times longer than wide. Metatibia strongly curved in dorsal view, slightly curved in lateral view. Number of spinules on dorsolateral margin of metatibia, proximal to row of stiff bristles, vary from seven to ten. In lateral view, first metatarsomere 0.55 – 0.57 times as long as metatibia. Proximal end of first metatarsomere ventrally with thick characteristic patch of short pointed and capitate setae in both sexes. Last male ventrite internally with longitudinal ridge along middle (Fig. 5); posterior margin bisinuate.
Aedeagus in lateral view gently curved, apex acutely pointed and slightly recurved (Fig. 4); ventral side depressed with transparent window, lateral edges raised (Fig. 2); dorsal opening covered with lamina not extending to apex (Fig. 3). Arms of tegmen subequal to or slightly shorter than stem.
Spermatheca with receptacle widest in middle, internal side strongly convex, external side weakly concave; pump with horizontal part longer than vertical; spermathecal duct curved towards receptacle, coiled thrice proximally, not reaching half of receptacle (Fig. 6). Vaginal palpus narrow at distal 1/3, widest at proximal 1/4; distal sclerotization shorter than proximal sclerotization or lateral membranous area (Fig. 8). Tignum nearly straight, anterior sclerotization slightly wider than posterior (Fig. 7).
Remarks
Longitarsus limnophilae can easily be separated from all other south Indian species of Longitarsus by the anteriorly widening, flat frontal ridge (in the other species, the frontal ridge is more or less narrowly raised). Longitarsus belgaumensis Jacoby closely resembles Longitarsus limnophilae in having narrowly piceous elytral suture and dark distal antennomeres. But Longitarsus limnophilae can be separated from Longitarsus belgaumensis based on the antenna extending slightly beyond the apex of elytra over pronotum (in Longitarsus belgaumensis, antenna does not extend beyond the apex of elytra over pronotum), pronotum anteriorly wider than posteriorly (in Longitarsus belgaumensis, pronotum is anteriorly as wide as posteriorly with the maximum width in the middle), structure of frontal ridge (frontal ridge sharply raised along middle in Longitarsus belgaumensis) and genitalia.
Host plants
Limnophila aquatica (Roxb.) Alston (Scrophulariaceae) (Fig. 10) is a rooted emergent hydrophyte growing in shallow streams, marshes and rice fields (Fig. 9). Species of Limnophila R. Br., 1810 are widely distributed in the tropics and subtropics of the Old World and also occur as weeds. Stem aerenchyma in Longitarsus aquatica resembles the same in Longitarsus sessiliflora (Vahl) Blume termed as “wheel-type” by Jung et al. (2008)[1]. Longitarsus limnophilae was also found to feed on Longitarsus repens (Benth.) Benth. (Fig. 11).
Biology
Eggs (Fig. 13) are laid on tender leaves and buds and the larvae are open feeders (Fig. 14). The closely oriented tender leaves provide sufficient cover for the larva. Mature larva enters the stem aerenchyma in the internode by boring a tiny hole and pupation occurs in it, a little above the entry hole (Figs 15, 16). Adult emerges through an exit hole nearly circular in shape with irregular margin. Adult feeds on both adaxial and abaxial surface of, mostly, tender leaves by scraping, often resulting in holes on the lamina (Fig. 12). Adults when thrown in water floated initially and then swam with raised antennae held back over the sides of the pronotum. After swimming for a while, some performed a short jump on to the shore.
Distribution
The types were collected from a single locality only. India (Kerala, Vembayam, 8°38'28"N, 76°56'39"E).
Discussion
Jolivet and Hawkeswood (1995)[2] have listed Bacopa Aublet, 1775 (Scrophulariaceae), a genus of hydrophytes, among host plants of Longitarsus. But no further information is available on its biology on Bacopa. Longitarsus limnophilae and species of Agasicles represent two independently evolved lineages in Alticini adapted for larval leaf feeding and pupation inside the stem. Agasicles and Longitarsus limnophilae are the only flea beetles known to pupate inside the stem of their aquatic host plant above the water level. Wheel shaped stem aerenchyma of Limnophila aquatica serve as a safe abode for the pupa offering protection against natural enemies. Species of Limnophila being widely distributed aquatic weeds, Longitarsus limnophilae could be a potential biocontrol agent for them.
This is the first report of an Oriental flea beetle pupating inside the stem of its hydrophyte host.
Original Description
- Prathapan, K; Viraktamath, C; 2011: A new species of Longitarsus Latreille, 1829 (Coleoptera, Chrysomelidae, Galerucinae) pupating inside stem aerenchyma of the hydrophyte host from the Oriental Region ZooKeys, 87: 1-10. doi
Other References
- ↑ Jung J, Lee S, Choi H (2008) Anatomical Patterns of Aerenchyma in Aquatic and Wetland Plants. Journal of Plant Biology 51:428-439.
- ↑ Jolivet P, Hawkeswood T (1995) Host-plants of Chrysomelidae of the world: An Essay about the Relationships between the Leaf-beetles and their Food-plants. Backhuys Publishers, Leiden, 281 pp.
Images
|