Gordius chiashanus
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Chiu2020ZooKeys941, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Chiu2020ZooKeys941">{{Citation See also the citation download page at the journal. |
Ordo: Gordea
Familia: Gordiidae
Genus: Gordius
Name
Gordius chiashanus Chiu sp. nov. – Wikispecies link – ZooBank link – Pensoft Profile
Type locality
Dinghu (23°29'29.10"N, 120°43'19.00"E), Alishan township, Chiayi county, Taiwan (holotype). Paratypes were collected from Dasyueshan (Heping district, Taichung city), Xitou (Lugu township, Nantou county), Shihjhuo, Fenqihu (Zhuqi township, Chiayi county), Dinghu (Alishan township, Chiayi county), and Hongshi forest road (Haituan township, Taitung county). Table 1 presents detailed information of the locality.
Type material
Partial bodies of the holotype and allotype were deposited at the National Museum of Natural Science, Taichung, Taiwan. Paratypes were deposited at the National Museum of Natural Science, Taichung, Taiwan and Lake Biwa Museum, Shiga, Japan (Table 1).
| Collection date | GenBank no. | Locality | Longitude and latitude | Collector | Depository | Sex | Status | Length (mm) |
|---|---|---|---|---|---|---|---|---|
| 20-XI-2017 | MN784831 1 | Dasyueshan (Heping, Taichung, Taiwan) | 24°14'47.90"N, 120°56'06.80"E | Ta-Chih Chen | NMNS | M | Free-living adult | 430 |
| 26-XI-2008 | MN784832 | Hongshi trail (Haituan, Taitung, Taiwan) | 23°04'14.50"N, 121°07'58.30"E | Po-Yen Chen | NMNS | M | Free-living adult | 744 |
| 22-I-2008 | MN784841 | Shihjhuo (Zhuqi, Chiayi, Taiwan) | 23°29'01.70"N, 120°42'05.90"E | Yu-Hsuan Tsai | NMNS | M | Free-living adult | 860 |
| 9-II-2007 | MN784833 | Shihjhuo (Zhuqi, Chiayi, Taiwan) | 23°29'01.70"N, 120°42'05.90"E | Yu-Hsuan Tsai | NMNS | F | Free-living adult | 707 |
| 8-XII-2017 | MN784819 | Dinghu (Alishan, Chiayi, Taiwan) | 23°29'29.10"N, 120°43'19.00"E | Ming-Chung Chiu | LBM | M | Free-living adult | 771 |
| 8-XII-2017 | MN784820 | Dinghu (Alishan, Chiayi, Taiwan) | 23°29'29.10"N, 120°43'19.00"E | Ming-Chung Chiu | NMNS | M | Free-living adult | 734 |
| 8-XII-2017 | MN784821 | Dinghu (Alishan, Chiayi, Taiwan) | 23°29'29.10"N, 120°43'19.00"E | Ming-Chung Chiu | NMNS | M | Free-living adult | 726 |
| 17-XII-2013 | MN784822 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Hua-Te Fang | LBM | M | Free-living adult | 803 |
| 17-XII-2013 | MN784823 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Hua-Te Fang | LBM | M | Free-living adult | 756 |
| 17-XII-2013 | MN784824 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Hua-Te Fang | NMNS | M | Free-living adult | 594 |
| 17-XII-2013 | MN784825 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Hua-Te Fang | NMNS | M | Free-living adult | 383 |
| 17-XII-2013 | MN784826 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Hua-Te Fang | NMNS | M | Free-living adult | 676 |
| 17-XII-2013 | MN784827 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Hua-Te Fang | NMNS | M | Free-living adult | 474 |
| 18-XII-2017 | MN784828 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Ming-Chung Chiu | NMNS | M | Free-living adult | 749 |
| 18-XII-2017 | MN784829 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Ming-Chung Chiu | NMNS | F | Free-living adult | 666 |
| 18-XII-2017 | MN784830 | Fenqihu (Zhuqi, Chiayi, Taiwan) | 23°30'12.70"N, 120°41'36.00"E | Ming-Chung Chiu | NMNS | F | Free-living adult | 717 |
| 18-XII-2016 | MN784816 | Xitou (Lugu, Nantou, Taiwan) | 23°40'21.30"N, 120°47'27.50"E | Ming-Chung Chiu | LBM | M | Free-living adult | 498 |
| 18-XII-2016 | MN784817 | Xitou (Lugu, Nantou, Taiwan) | 23°40'21.30"N, 120°47'27.50"E | Ming-Chung Chiu | NMNS | M | Free-living adult | 403 |
| 18-XII-2016 | MN784818 | Xitou (Lugu, Nantou, Taiwan) | 23°40'21.30"N, 120°47'27.50"E | Ming-Chung Chiu | LBM | F | Free-living adult | 549 |
| 9-II-2008 | MN784842 | Xitou (Lugu, Nantou, Taiwan) | 23°40'21.30"N, 120°47'27.50"E | Ming-Chung Chiu | NMNS | M | Free-living adult | 572 |
| 10-XII-2011 | MN784840 | Xitou (Lugu, Nantou, Taiwan) | 23°40'21.30"N, 120°47'27.50"E | Ming-Chung Chiu | NMNS | M | Free-living adult | 502 |
| 17-III-2019 | MN784839 | Xitou (Lugu, Nantou, Taiwan) | 23°40'21.30"N, 120°47'27.50"E | Zhao-Hui Lin | NMNS | - | Dead worm in host | - |
| 23-VII-2018 | MN784834 | Shihjhuo (Zhuqi, Chiayi, Taiwan) | 23°29'01.70"N, 120°42'05.90"E | Yu-Wei Li | NMNS | - | Immature worm | 660 |
| 28-VII-2018 | MN784835 | Shihjhuo (Zhuqi, Chiayi, Taiwan) | 23°28'22.60"N, 120°41'42.80"E | Yu-Wei Li | NMNS | - | Immature worm | 894 |
| 28-VII-2018 | MN784836 | Shihjhuo (Zhuqi, Chiayi, Taiwan) | 23°28'22.60"N, 120°41'42.80"E | Yu-Wei Li | NMNS | - | Immature worm | 420 |
| 28-VII-2018 | MN784837 | Shihjhuo (Zhuqi, Chiayi, Taiwan) | 23°28'22.60"N, 120°41'42.80"E | Yu-Wei Li | NMNS | - | Immature worm | 442 |
| 28-VII-2018 | MN784838 | Shihjhuo (Zhuqi, Chiayi, Taiwan) | 23°28'22.60"N, 120°41'42.80"E | Yu-Wei Li | NMNS | - | Immature worm | 426 |
Type hosts
Spirobolus sp. nov. (Hsu and Chang, unpublished) (Diplopoda: Spirobolidae) (Fig. 5E, F)
Etymology
The specific name is the combination of chia, referring to the place (Chiayi county) where the first sample was found, and shan, referring to the Chinese word for “mountains.” The word chia is also in memory of our friend, Chia-Chih Lin, who died in an accident in a field experiment.
Description
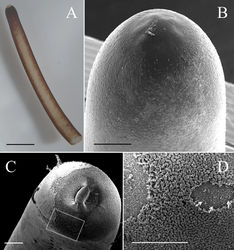
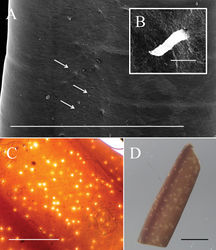
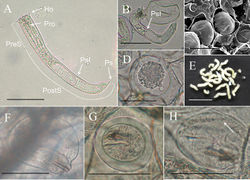
Male adults (N = 11) (Figs 1–3, 5). Body length 627.94 ± 154.75 (383–860) mm, width (widest, after dehydration) 1.30 ± 0.31 (0.81–2.06) mm, light to dark brown, smooth, and covered with mucus-like structure (viscous liquid on live worms with rainbow-like reflection (Fig. 5C, Suppl. material 1: Video S1), and created haze that surrounded the body surface in hot water (Fig. 5A). Anterior end columnar and spherical; anterior tip white (white cap) with a dark -brown collar and a vertical white stripe on the ventral side (Fig. 1A). Under SEM, surface of anterior end appeared smooth (Fig. 1B) or wrinkled (Fig. 1C) on the tip of one sample; scattered short bristles (11.24 ± 6.57 (4.92–22.24) µm in length) scattered except on tip in most samples (Fig. 1B, D).
Cuticle in mid-body ornamented with a dorsal and a ventral dark pigment line; white spots scattered across entire body surface (Figs 3C, D, 5A). Under SEM, cuticle surface appeared smooth (Fig. 3A) with a few scattered short or cone-like bristles (6.75 ± 2.37 (2.31–10.34) µm in length) (Fig. 3A, B).
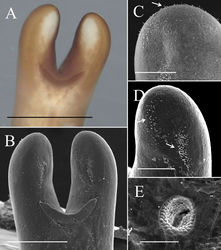
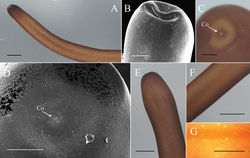
Posterior end divided into two tail lobes (Fig. 2A, B), each lobe 855.24 ± 100.89 (658.39–994.88) µm long and 458.55 ± 76.52 (365.95–643.00) µm wide with length-to-width ratio of 1.89 ± 0.26 (1.49–2.42). Inner side of lobe tips white (Fig. 2A). Under SEM, inner side of tail lobes concave in some samples; cuticle surface smooth, but one sample exhibited flat areoles on inner side of lobe tips; short bristles scattered across the surface and concentrated in most samples on lobe tips (Fig. 2C) and on inner side of lobe tips forming a bristle field (322.67 ± 99.34 (187.60–412.75) µm long and 71.82 ± 35.49 (44.81–114.54) µm wide) on each of tail lobe posterior to tips of postcloacal crescent (Fig. 2D). Postcloacal crescent (Fig. 2A, B) 718.61 ± 118.77 (536.14–984.34) µm long and 86.7 ±15.62 (54.73–118.65) µm wide and located on ventral side near base of tail lobes. Crescent generally semicircular or slightly angled, but a few samples exhibited a straightened form of crescent. Branches of postcloacal crescent usually ended at tail lobes. Cloacal opening circular (40.5 ± 21.87 (27.41–56.14) µm) and anterior to postcloacal crescent (Fig. 2A, B). Wall inside cloacal opening exhibited areoles (Fig. 2E); no circumcloacal spine or bristles observed in region next to cloacal opening. Female adults (N = 4) (Figs 4, 5). Body length 659.75 ± 77.06 (549–717) mm, width (widest, after dehydration) 1.54 ± 0.54 (1.00–2.03) mm, light to dark brown, smooth, and covered with mucus-like structure. White spots scattered on surface but relatively less obvious than those of male adults (Fig. 4F, G). Anterior end columnar and spherical. Anterior tip white (white cap) with a dark-brown collar and exhibited a vertical white stripe on the ventral side (Fig. 4A). Under SEM, surface of anterior end smooth and exhibited scattered short bristles (16.75 ± 4.60 (13.39–23.56) µm in length) except at tip (Fig. 4B). Cuticle in mid-body ornamented with a dorsal and a ventral dark pigment line (Fig. 4G). Under SEM, cuticle surface smooth with a few short or cone-like bristles (7.24 ± 2.01 (4.94–9.99) µm in length) scattered. Posterior end columnar and rounded at tip (Fig. 4E) and did not exhibit scattered bristles (Fig. 4D). Cloacal opening on terminal end (Fig. 4C, D) circular and 36.56 ± 23.23 (24.68–48.45) µm in diameter. Eggs (N = 12) (Fig. 6C–E). Egg strings (Fig. 6E) 7.41 ± 3.46 (3.78–13.70) mm in length and 1.13 ± 0.12 (0.86–1.25) mm in width; white or light yellow in color, deposited in water as short pieces not adhering to substrate. Eggs round, 54.16 ± 242 2.89 (49.88–58.61) µm in diameter. Developing embryo surrounded by an inner membrane (Fig. 6C, D) separated by a distinct space from outer egg shell 14.35 ± 1.41 (12.43–17.33) µm).
Living larvae (N = 10) (Fig. 6B). Eggs developed for approximately 49 days. Hatched larvae remained near egg strings or moved inside eggshells. Under light microscopy, living larvae appeared cylindrical with a single posterior spine. Preseptum length 32.33 ± 4.53 (27.06–40.04) µm, and the width 18.04 ± 0.86 (16.70–19.12) µm. Postseptum length 83.05 ± 8.31 (66.50–92.66) µm, width 15.05 ± 0.73 (14.21–16.10) µm; proboscis length 14.94 ± 1.99 (12.35–18.48) µm, width 4.11 ± 0.85 (2.77–5.34) µm; pseudointestine length 60.60 ± 5.40 (54.99–70.12) µm, width 11.66 ± 1.42 (8.84–13.56) µm, unequally subdivided, elongated oval with a depression in anterior end (Fig. 6B).
Larvae treated with hot water (N = 2) (Fig. 6A). Larvae treated with hot water similar in morphology but larger than living larvae. Preseptum length 44.57 ± 0.13 (44.48–44.66) µm, width 17.96 ± 0.16 (17.85–18.08) µm. Postseptum length 118.23 ± 1.91 (116.88–119.58) µm, width 15.36 ± 0.68 (14.88–15.84) µm. Proboscis length 12.63 ± 1.18 (11.80–13.47) µm, width 3.26 ± 0.05 (3.23–3.30) µm; pseudointestine length 77.99 ± 5.22 (74.30–81.68) µm, width 13.99 ± 0.81 (13.41–14.56) µm (Fig. 6A).
Field-collected cysts (N = 5) (Fig. 6F–H) . Larvae in cysts unfolded (N = 4) (Fig. 6F) or exhibited a postseptum folded twice (N = 1) (Fig. 6G, H). Unfolded larvae morphologically similar to larvae but larger in size; preseptum length was 60.18 ± 6.72 (50.40–65.18) µm, width 20.87 ± 0.52 (20.28–21.33) µm; postseptum length 127.33 ± 20.05 (105.10–146.05) µm, width 19.82 ± 2.27 (17.61–22.91) µm; proboscis length 15.46 ± 1.67 (13.84–17.56) µm, width 4.10 ± 0.68 (3.09–4.52) µm; pseudointestine not visible (Fig. 6F). Folded larva (length 34.97 µm, width 30.47 µm) fold twice and surrounded by a clear cyst wall, 47.86 µm in total length and 42.40 µm in total width; proboscis length 15.57 µm, width 5.09 µm (Fig. 6G); a single posterior spine visible after treatment with a solution of 5% KOH (Fig. 6H).
| Accession number | Species/clade | Reference |
|---|---|---|
| Gordius/Acutogordius | ||
| KM382317 | G. cf. robustus (Clade 8) | Hanelt et al. 2015[1] |
| KM382316 | ‘’ | Hanelt et al. 2015[1] |
| KM382315 | ‘’ | Hanelt et al. 2015[1] |
| KM382314 | ‘’ | Hanelt et al. 2015[1] |
| KM382313 | ‘’ | Hanelt et al. 2015[1] |
| KM382312 | ‘’ | Hanelt et al. 2015[1] |
| KM382311 | ‘’ | Hanelt et al. 2015[1] |
| KM382310 | G. terrestris | Hanelt et al. 2015[1], Anaya et al. 2019[2] |
| KM382309 | ‘’ | Hanelt et al. 2015[1], Anaya et al. 2019[2] |
| KM382308 | ‘’ | Hanelt et al. 2015[1], Anaya et al. 2019[2] |
| KM382307 | ‘’ | Hanelt et al. 2015[1], Anaya et al. 2019[2] |
| KM382306 | G. cf. robustus (Clade 6) | Hanelt et al. 2015[1] |
| KM382305 | ‘’ | Hanelt et al. 2015[1] |
| KM382304 | ‘’ | Hanelt et al. 2015[1] |
| KM382303 | ‘’ | Hanelt et al. 2015[1] |
| KM382302 | ‘’ | Hanelt et al. 2015[1] |
| KM382301 | ‘’ | Hanelt et al. 2015[1] |
| KM382300 | ‘’ | Hanelt et al. 2015[1] |
| KM382299 | ‘’ | Hanelt et al. 2015[1] |
| KM382297 | G. cf. robustus (Clade 5) | Hanelt et al. 2015[1] |
| KM382296 | ‘’ | Hanelt et al. 2015[1] |
| KM382295 | ‘’ | Hanelt et al. 2015[1] |
| KM382294 | G. cf. robustus (Clade 4) | Hanelt et al. 2015[1] |
| KM382293 | ‘’ | Hanelt et al. 2015[1] |
| KM382292 | ‘’ | Hanelt et al. 2015[1] |
| KM382291 | ‘’ | Hanelt et al. 2015[1] |
| KM382290 | ‘’ | Hanelt et al. 2015[1] |
| KM382289 | G. cf. robustus (Clade 3) | Hanelt et al. 2015[1] |
| KM382288 | ‘’ | Hanelt et al. 2015[1] |
| KM382287 | ‘’ | Hanelt et al. 2015[1] |
| KM382286 | ‘’ | Hanelt et al. 2015[1] |
| KM382285 | ‘’ | Hanelt et al. 2015[1] |
| KM382284 | ‘’ | Hanelt et al. 2015[1] |
| KM382283 | G. cf. robustus (Clade 2) | Hanelt et al. 2015[1] |
| KM382282 | ‘’ | Hanelt et al. 2015[1] |
| KM382281 | G. cf. robustus (Clade 1) | Hanelt et al. 2015[1] |
| KM382280 | ‘’ | Hanelt et al. 2015[1] |
| KM382279 | ‘’ | Hanelt et al. 2015[1] |
| KM382278 | ‘’ | Hanelt et al. 2015[1] |
| KM382277 | ‘’ | Hanelt et al. 2015[1] |
| KM382318 | G. attoni | Hanelt et al. 2015[1] |
| KM382319 | ‘’ | Hanelt et al. 2015[1] |
| KM382320 | G. balticus | Hanelt et al. 2015[1] |
| KM382321 | Gordius sp. N178 | Hanelt et al. 2015[1] |
| KM382322 | Gordius sp. N183 | Hanelt et al. 2015[1] |
| KM382323 | Gordius sp. N297B | Hanelt et al. 2015[1] |
| KM382324 | Gordius sp. N357 | Hanelt et al. 2015[1] |
| AB647235 | Gordius sp. KW-2011-A | Sato et al. 2012[3] |
| AB647237 | Gordius sp. KW-2011-B | Sato et al. 2012[3] |
| AB647241 | Gordius sp. KW-2011-D | Sato et al. 2012[3] |
| KY172751 | Gordius sp. Tobias et al. 2017[4] | Tobias et al. 2017[4] |
| KY172750 | ‘’ | Tobias et al. 2017[4] |
| KY172752 | ‘’ | Tobias et al. 2017[4] |
| KY172759 | ‘’ | Tobias et al. 2017[4] |
| KY172765 | ‘’ | Tobias et al. 2017[4] |
| KY172770* | ‘’ | Tobias et al. 2017[4] |
| KY172777 | ‘’ | Tobias et al. 2017[4] |
| KY172749 | ‘’ | Tobias et al. 2017[4] |
| KY172792 | ‘’ | Tobias et al. 2017[4] |
| KY172789 | ‘’ | Tobias et al. 2017[4] |
| KY172791 | ‘’ | Tobias et al. 2017[4] |
| KY172799 | ‘’ | Tobias et al. 2017[4] |
| KY172801 | ‘’ | Tobias et al. 2017[4] |
| KY172802 | ‘’ | Tobias et al. 2017[4] |
| KY172804 | ‘’ | Tobias et al. 2017[4] |
| KY172753 | G. paranensis (Clade2) | Tobias et al. 2017[4] |
| KY172754 | ‘’ | Tobias et al. 2017[4] |
| KY172755 | ‘’ | Tobias et al. 2017[4] |
| KY172756 | ‘’ | Tobias et al. 2017[4] |
| KY172776 | ‘’ | Tobias et al. 2017[4] |
| KY172782 | ‘’ | Tobias et al. 2017[4] |
| KY172813 | ‘’ | Tobias et al. 2017[4] |
| KY172811 | G. paranensis (Clade1) | Tobias et al. 2017[4] |
| KY172812 | ‘’ | Tobias et al. 2017[4] |
| KX591948 | Acutogordius taiwanensis | Chiu et al. 2017[5] |
| KX591947 | ‘’ | Chiu et al. 2017[5] |
| KX591946 | ‘’ | Chiu et al. 2017[5] |
| KX591945 | ‘’ | Chiu et al. 2017[5] |
| KX591944 | ‘’ | Chiu et al. 2017[5] |
| KX591943 | ‘’ | Chiu et al. 2017[5] |
| KX591942 | ‘’ | Chiu et al. 2017[5] |
| KX591941 | ‘’ | Chiu et al. 2017[5] |
| KX591940 | ‘’ | Chiu et al. 2017[5] |
| KX591939 | ‘’ | Chiu et al. 2017[5] |
| KX591938 | ‘’ | Chiu et al. 2017[5] |
| KX591937 | ‘’ | Chiu et al. 2017[5] |
| KX591936 | ‘’ | Chiu et al. 2017[5] |
| KX591935 | ‘’ | Chiu et al. 2017[5] |
| KX591934 | ‘’ | Chiu et al. 2017[5] |
| KX591933 | ‘’ | Chiu et al. 2017[5] |
| KX591932 | ‘’ | Chiu et al. 2017[5] |
| KX591931 | ‘’ | Chiu et al. 2017[5] |
| KX591930 | ‘’ | Chiu et al. 2017[5] |
| KX591929 | ‘’ | Chiu et al. 2017[5] |
| KX591928 | ‘’ | Chiu et al. 2017[5] |
| KX591927 | ‘’ | Chiu et al. 2017[5] |
| KX591926 | ‘’ | Chiu et al. 2017[5] |
| KX591925 | ‘’ | Chiu et al. 2017[5] |
| KX591924 | ‘’ | Chiu et al. 2017[5] |
| KX591923 | ‘’ | Chiu et al. 2017[5] |
| KX591922 | ‘’ | Chiu et al. 2017[5] |
| MF983649 | Myanmar nematomorph | |
| Out group | ||
| HM044105 | Chordodes formosanus | Chiu et al. 2011[6] |
| HM044124 | ‘’ | Chiu et al. 2011[6] |
| KY172780 | Euchordodes nigromaculatus | Tobias et al. 2017[4] |
| KY172803 | ‘’ | Tobias et al. 2017[4] |
| KY172747 | Parachordodes diblastus | Tobias et al. 2017[4] |
| KY172778 | ‘’ | Tobias et al. 2017[4] |
Phylogeny
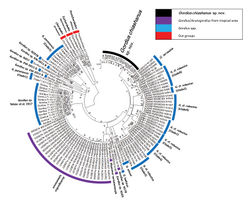
The partial COI sequences of the 18 free-living adults contained 15 haplotypes with 392 invariable sites, nine singletons, and 21 parsimoniously informative sites. The genetic distance among them was 0.0024 within the range of 0.0000–0.0510. The three living adults and six worms inside the hosts were considered conspecific with the 18 free-living adults because of their small genetic distances (0.0000–0.0719). The mean interspecific genetic distances between Gordius chiashanus sp. nov. and other Gordius species or clades were in the range of 0.2320–0.4242, and that between Gordius chiashanus sp. nov. and Acutogordius taiwanensis was 0.3648 (Table 3). In addition to short genetic distances, the conspecific status of the 18 free-living adults was also supported because all the samples were located in a single clade, as indicated by a high bootstrap value. No subgroup was detected because the polytomic topology exhibited low bootstrap values and short genetic distances. The Gordius species/clades in the present result were consistent with the results of Hanelt et al. (2015)[1] and Tobias et al. (2017)[4], despite slight differences in the relative relationships among species, which might be attributable to the differences in models used or the shorter sequence adopted in previous studies. The clade of A. taiwanensis was located within that of the Gordius species, and it did not behave as a sister group (Fig. 7).
Reproductive season
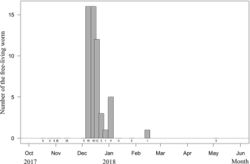
Free-living adult worms frequently aggregate and mate on wet ground (Fig. 5B, C) after rain or fog, and they are sometimes found in water or soil (Fig. 5D). They suddenly emerge in early December, and their number decreases within 1–2 months (Fig. 8). During the reproductive season, no infected host was found. The seasonality and pattern of Gordius chiashanus sp. nov. differed from the graph constructed using data from C. formosanus (Chiu et al. 2016[7]).
Diagnosis and comments
The 21 free-living Gordius adults and six juvenile worms from round-backed millipedes were judged as belonging to the same species in accordance with the results that they all were located in the same clade in the phylogenetic tree and had low genetic distances (Fig. 7, Table 3). These samples were regarded as a new species, Gordius chiashanus sp. nov., on the basis of their distribution patterns of bristles on the male tail and presence of a vertical white stripe on the anterior ventral side and areoles on the inside wall of the cloacal opening.
| Species/Clade | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gordius chiashanus sp. nov. | 0.024 | |||||||||||||||||||||
| 2 | G. cf. robustus (Clade1) | 0.285 | 0.009 | ||||||||||||||||||||
| 3 | G. cf. robustus (Clade2) | 0.312 | 0.217 | 0.015 | |||||||||||||||||||
| 4 | G. cf. robustus (Clade3) | 0.293 | 0.297 | 0.275 | 0.007 | ||||||||||||||||||
| 5 | G. cf. robustus (Clade4) | 0.308 | 0.208 | 0.249 | 0.157 | 0.012 | |||||||||||||||||
| 6 | G. cf. robustus (Clade5) | 0.272 | 0.165 | 0.211 | 0.227 | 0.222 | 0.003 | ||||||||||||||||
| 7 | G. cf. robustus (Clade6) | 0.293 | 0.257 | 0.251 | 0.255 | 0.228 | 0.259 | 0.006 | |||||||||||||||
| 8 | G. terrestris | 0.232 | 0.209 | 0.265 | 0.250 | 0.230 | 0.222 | 0.238 | 0.020 | ||||||||||||||
| 9 | G. cf. robustus (Clade8) | 0.265 | 0.203 | 0.307 | 0.338 | 0.253 | 0.244 | 0.251 | 0.122 | 0.026 | |||||||||||||
| 10 | G. attoni | 0.277 | 0.229 | 0.288 | 0.337 | 0.274 | 0.238 | 0.289 | 0.231 | 0.249 | 0.010 | ||||||||||||
| 11 | G. balticus | 0.316 | 0.260 | 0.298 | 0.288 | 0.269 | 0.274 | 0.304 | 0.264 | 0.323 | 0.337 | – | |||||||||||
| 12 | Gordius sp. N178 | 0.352 | 0.260 | 0.313 | 0.370 | 0.289 | 0.340 | 0.330 | 0.256 | 0.290 | 0.271 | 0.323 | – | ||||||||||
| 13 | Gordius sp. N183 | 0.329 | 0.302 | 0.290 | 0.373 | 0.317 | 0.344 | 0.365 | 0.294 | 0.336 | 0.277 | 0.301 | 0.246 | – | |||||||||
| 14 | Gordius sp. N297B | 0.424 | 0.416 | 0.462 | 0.547 | 0.441 | 0.443 | 0.478 | 0.375 | 0.412 | 0.348 | 0.455 | 0.343 | 0.414 | – | ||||||||
| 15 | Gordius sp. N357 | 0.332 | 0.366 | 0.387 | 0.420 | 0.376 | 0.396 | 0.302 | 0.357 | 0.359 | 0.379 | 0.439 | 0.375 | 0.434 | 0.441 | – | |||||||
| 16 | Gordius sp. KW-2011-A | 0.384 | 0.325 | 0.327 | 0.453 | 0.371 | 0.336 | 0.345 | 0.347 | 0.348 | 0.331 | 0.376 | 0.332 | 0.376 | 0.372 | 0.424 | – | ||||||
| 17 | Gordius sp. KW-2011-B | 0.334 | 0.375 | 0.365 | 0.370 | 0.334 | 0.407 | 0.364 | 0.302 | 0.363 | 0.333 | 0.380 | 0.323 | 0.358 | 0.333 | 0.308 | 0.290 | – | |||||
| 18 | Gordius sp. KW-2011-D | 0.375 | 0.300 | 0.344 | 0.393 | 0.373 | 0.294 | 0.388 | 0.388 | 0.367 | 0.369 | 0.405 | 0.384 | 0.390 | 0.374 | 0.403 | 0.312 | 0.301 | – | ||||
| 19 | G. paranensis (Clade1) | 0.369 | 0.405 | 0.381 | 0.450 | 0.381 | 0.410 | 0.359 | 0.373 | 0.395 | 0.409 | 0.398 | 0.408 | 0.466 | 0.426 | 0.453 | 0.415 | 0.386 | 0.440 | 0.049 | |||
| 20 | G. paranensis (Clade2) | 0.337 | 0.348 | 0.391 | 0.436 | 0.384 | 0.372 | 0.368 | 0.333 | 0.368 | 0.345 | 0.339 | 0.334 | 0.385 | 0.324 | 0.404 | 0.357 | 0.327 | 0.344 | 0.377 | 0.010 | ||
| 21 | Gordius sp. Tobias et al. 2017[4] | 0.335 | 0.283 | 0.293 | 0.436 | 0.355 | 0.311 | 0.366 | 0.287 | 0.337 | 0.347 | 0.358 | 0.254 | 0.308 | 0.343 | 0.353 | 0.304 | 0.335 | 0.321 | 0.354 | 0.337 | 0.012 | |
| 22 | Acutogordius taiwanensis | 0.365 | 0.343 | 0.327 | 0.401 | 0.386 | 0.368 | 0.345 | 0.322 | 0.375 | 0.304 | 0.336 | 0.270 | 0.210 | 0.462 | 0.469 | 0.432 | 0.376 | 0.366 | 0.435 | 0.389 | 0.311 | 0.002 |
Although the distribution pattern of the bristles is similar to that of G. helveticus, G chiashanus sp. nov. is morphologically distinct because of the presence of stout bristles on the mid-body, a vertical white stripe on the anterior ventral side, and areoles on the inside wall of the cloacal opening. The vertical white stripe on the anterior ventral side can be easily observed by the naked eye, but it has rarely been mentioned thus far. The presence of a white stripe was previously reported in the terrestrial hairworm, G. terrestris (Anaya et al. 2019[2]), which exhibits a broad white patch; however, the patch is likely to be the intensive aggregation of white spots in Gordius chiashanus sp. nov. The presence of areoles on the inside wall of the cloacal opening has only been reported in an unknown Gordius (Schmidt-Rhaesa 2012[10], fig. 3.2.2). Although cloacal openings are usually covered by contamination in many Gordius species, as was the case in most of our samples, the areole on the inside wall of cloacal opening might not be a general characteristic of the genus Gordius because it is absent in at least some species (e.g., G. serratus Schmidt-Rhaesa, 2010, G. terrestris, G. spiridonovi) (Schmidt-Rhaesa 2010[9]; Schmidt-Rhaesa and Prous 2010[11]; Anaya et al. 2019[2]).
Original Description
- Chiu, M; Huang, C; Wu, W; Lin, Z; Chen, H; Shiao, S; 2020: A new millipede-parasitizing horsehair worm, Gordius chiashanus sp. nov., at medium altitudes in Taiwan (Nematomorpha, Gordiida) ZooKeys, 941: 25-48. doi
Images
|
Other References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 1.36 1.37 1.38 1.39 1.40 1.41 1.42 1.43 1.44 1.45 1.46 1.47 Hanelt B, Schmidt-Rhaesa A, Bolek M (2015) Cryptic species of hairworm parasites revealed by molecular data and crowdsourcing of specimen collections.Molecular Phylogenetics and Evolution82: 211–218. https://doi.org/10.1016/j.ympev.2014.09.010
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Anaya C, Schmidt-Rhaesa A, Hanelt B, Bolek M (2019) A new species of Gordius (Phylum Nematomorpha) from terrestrial habitats in North America.ZooKeys892: 59–75. https://doi.org/10.3897/zookeys.892.38868
- ↑ 3.0 3.1 3.2 Sato T, Watanabe K, Tamotsu S, Ichikawa A, Schmidt-Rhaesa A (2012) Diversity of nematomorph and cohabiting nematode parasites in riparian ecosystems around the Kii Peninsula, Japan.Canadian Journal of Zoology90: 829–838. https://doi.org/10.1139/z2012-048
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 4.21 4.22 4.23 4.24 4.25 4.26 4.27 4.28 4.29 4.30 Tobias Z, Yadav A, Schmidt-Rhaesa A, Poulin R (2017) Intra- and interspecific genetic diversity of New Zealand hairworms (Nematomorpha).Parasitology144: 1026–1040. https://doi.org/10.1017/S0031182017000233
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 5.25 5.26 Chiu M, Huang C, Wu W, Shiao S (2017) A new orthopteran-parasitizing horsehair worm, Acutogordius taiwanensis sp. n., with a redescription of Chordodes formosanus and novel host records from Taiwan (Nematomorpha, Gordiida).ZooKeys683: 1–23. https://doi.org/10.3897/zookeys.683.12673
- ↑ 6.0 6.1 Chiu M, Huang C, Wu W, Shiao S (2011) A new horsehair worm, Chordodes formosanus sp. n. (Nematomorpha, Gordiida) from Hierodula mantids of Taiwan and Japan with redescription of a closely related species, Chordodes japonensis.ZooKeys160: 1–22. https://doi.org/10.3897/zookeys.160.2290
- ↑ Chiu M, Huang C, Wu W, Shiao S (2016) Annual survey of horsehair worm cysts in northern Taiwan, with notes on a single seasonal infection peak in chironomid larvae (Diptera: Chironomidae).Journal of Parasitology102: 319–326. https://doi.org/10.1645/15-907
- ↑ 8.0 8.1 Spiridonov S (1984) Two new Nematomorpha species of the family Gordiidae.Proceedings of the Zoological Institute USSR Academy of Sciences126: 97–101.
- ↑ 9.0 9.1 9.2 9.3 Schmidt-Rhaesa A (2010) Considerations on the genus Gordius (Nematomorpha, horsehair worms), with the description of seven new species.Zootaxa2533: 1–35. https://doi.org/10.11646/zootaxa.2533.1.1
- ↑ Schmidt-Rhaesa A (2012) Nematomorpha. In: Schmidt-Rhaesa A (Ed.) Handbook of Zoology.Gastrotricha, Cycloneuralia and Gnathifera (Vol. 1). Nematomorpha, Priapulida, Kinorhyncha and Loricifera. De Gruyter, Berlin, 29–145. https://doi.org/10.1515/9783110272536
- ↑ Schmidt-Rhaesa A, Prous M (2010) Records of horsehair worms (Nematomorpha) in Estonia, with description of three new species from the genus Gordius L.Estonian Journal of Ecology59: 39–51. https://doi.org/10.3176/eco.2010.1.03