Epimartyria
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Davis2012ZooKeys183, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Davis2012ZooKeys183">{{Citation See also the citation download page at the journal. |
Ordo: Lepidoptera
Familia: Micropterigidae
Name
Epimartyria Walsingham – Wikispecies link – Pensoft Profile
- Epimartyria Walsingham, 1898: 161.– Kearfott in Smith 1903[1]: 125.– Dyar 1903[2]: 581.– Meyrick 1912[3]: 3.– Forbes 1923[4]: 63.- McDunnough 1939[5]: 2.– Davis 1983[6]: 5; 1987[7]: 341.– Kristensen 1984b[8]: 97.– Nye and Fletcher 1991[9]: 113.– Poole 1996[10]: 716.– Hashimoto 2006[11]: 98.
- Micropteryx Hübner.– Forbes 1923[4]: 64 (subgenus Epimartyria Walsingham).
Type species
Micropteryx pardella Walsingham, by original designation.
Diagnosis
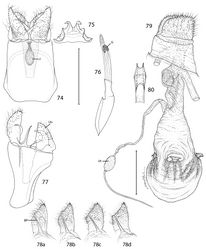
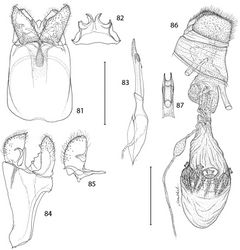
Epimartyria appears closely allied to the Asian genus Paramartyria as suggested by the similar elongate process arising from the inner base of the male valvae (Fig. 78) and by similar larval chaetotaxy (Hashimoto 2006[11]). More significantly, close affinities of these two northern genera were also indicated from the molecular study initiated by Kobayashi et al. (2000)[12] and Gibbs et al. (2004)[13], based on the 16S rRNA gene. At least one species of the Asian genus Vietomartyria, Vietomartyria nankushana Hirowatari & Hashimoto (Hirowatari et al. 2009[14]), also posseses a similar basal process on the valva, as pointed out by one reviewer. The forelegs of Vietomartyria also lack an epiphysis as do two species of Epimartyria. Epimartyria differs from all other micropterigid genera in possessing a deeply divided phallus, and from Paramartyria in particular by possessing a pair of lateral projections near the apical one third of the distal phallus and in having tergum X divided into dorsal and ventral processes (Hashimoto 2006[11]). Hashimoto (2006)[11] also mentioned the presence of an epiphysis in Paramartyria as one feature that distinguishes the latter from Epimartyria. Two of the three species of Epimartyria lack an epiphysis, but an epiphysis is present in Epimartyria pardella (Fig. 17).
Adult
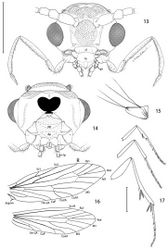
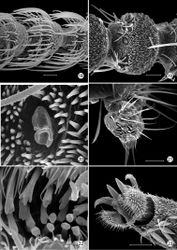
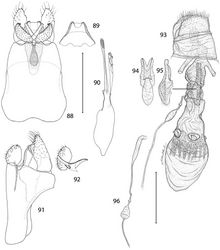
Head (Figs 13–15): Vestiture entirely hairy, scales erect and piliform with acute apices. Antenna (Figs 18–20) 0.75–0.9× length of forewing, slightly longer in male; pedicel enlarged, ~ 1.5× length of first flagellomere; flagellum moniliform, with 46–58 flagellomeres in male, 38–47 in female; flagellomeres mostly sparsely covered with long, piliform scales which exceed the length of their supporting flagellomere; basal 2–3 flagellomeres in male and 5–7 in female covered dorsally with moderately broad scales; a pair of large ascoid sensilla, opposite one another, with ~ 11–16 elongate, curved, sensory branches (Fig. 18) on each flagellomere; a single irregularly shaped and often bilobed multiporus sensillum placodeum (Faucheux 1997[15]) arising between the ascoid sensillae from a shallow pit near the ventral anterior margin of the flagellomere. Compound eyes reduced, interocular index (Davis 1975[16]) ~ 0.35–0.37; interfacetal setae absent. Ocelli present, base moderately elevated. Labrum approximately pentagonal, length ~ 2× that of clypeus. Mandible elongate triangular in form; distal edge truncate. Maxillary palpus elongate, 5-segmented, with main flexions between segments 1 and 2 and between 3 and 4; length ratio from basal segment 1: 1:2.7:2.7:0.9. Labial palpus short, total length ~ equal to that of basal segment of maxillary palpus; 2- segmented; sensory pit (organ vom Rath) present distally on apical segment enclosing numerous sensillae; apices of most sensillae terminating in a cluster of ~ 2- 5 minute acute lobes (Figs 21–22). Proximal prelabial sclerite slender, crescentiform; distal prelabial sclerite broadly triangular. Occipital sulcus incomplete but distinct laterally.
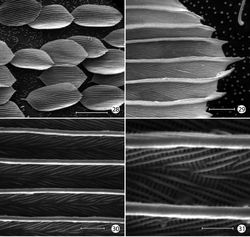
Thorax: Scales of mesonotum broad, appressed. Metanotum mostly naked except for a few long, piliform scales. Tegulae rather sparsely covered with long piliform scales. Forewing length: 4.2–5.5 mm; forewing (Fig. 16) with humeral vein present; Sc deeply bifurcate; R simple; Sc-R crossvein present near fork of Sc; Rs with 4 veins; Rs3–4 fused to ~ basal 1/3; accessory cell present; M with 3 branches; 1A and 2A fused over distal half; 3A extending across base of moderately small jugal lobe. Wing scale morphology of the primitive, generally non-glossatan type (Kristensen and Simonsen 2003[17]) consisting of fused dorsal and ventral surfaces (without internal chambers), and with a herringbone pattern formed by oblique-longitudinal crests overlying a dense layer of transverse microribs (Figs 28–31). Hindwing venation similar to forewing except with Sc and R fused; 1A and 2A completely fused; anal crossvein connecting to CuP near distal 2/3; scales over distal third of hindwing dark fuscous and nearly as broad and iridescent as in forewing; scales gradually becoming more slender, gray, and without iridescence over basal 2/3. Legs (Fig. 17) with tibial spur pattern of 0–0-4; a short epiphysis ~ 1/3 the length of tibia arising slightly beyond its midlength present in Epimartyria pardella; epiphysis absent in Epimartyria auricrinella and bimaculella; pretarsus (Figs 24–27) consisting of a pair of strongly curved claws; a lateral pair of pad-like pulvilli densely covered with long spinose setae; a median arolium with apical surface densely lined with minute grooves (Fig. 27); pseudempodial seta (Fig. 25) with longitudinal grooves.
Abdomen: Cuticle dark brown, sparsely covered with long, piliform scales. A pair of glands present, opening on sternum V in both sexes (Philpott 1925[18]); glands similar to those present in Paleomicroides, Paramartyria, and Neomicropteryx in not protruding and possessing a narrow slit-like opening within a smooth, hyaline area (Kristensen 1984a[19]).
Male genitalia: Tergum X (uncus) ~ half the median ventral length of IX; apex deeply divided nearly half its length into two broad lobes. Sternum X (venter X) variously bilobed, with or without short lateral lobes. Segment IX a completely sclerotized ring, with dorsal median length ~ 1/6 of ventral length. Sternum IX (vinculum) a broad plate with subparallel lateral margins; anterior end as broad or broader than caudal end. Valva with a subacute to rounded apex; base of valva with a long digitate process from mesal surface. Medial plate (juxta) with a slender stalk-like base gradually expanding anteriorly to a small, flat, oval plate. Distal phallus divided into two slender branches ~ half the total length of phallus; shorter dorsal branch of phallus terminating in gonopore (phallotreme) with thickened radial folds; a pair of minute, acute spines present laterally near distal third of dorsal branch; apex of longer ventral branch densely covered with numerous minute flattened scutate processes with rounded apices directed basad; phallobase moderately inflated, as long as or slightly longer than divided branches.
Female genitalia: Abdominal segment IX a complete ring with mid dorsal length ~ 0.5–0.6× the mid ventral length. Segment X consisting of a pair of lateral, setose plates; cloaca ending terminally; X often telescoped into IX and VIII in repose. Apophyses absent. Genital chamber with thickened walls surrounding a variably shaped slerite; caudal end of sclerite furcate. Ductus spermatheca with a moderately enlarged, spindle-shaped reservoir (utriculus) located at varying distances along ductus. Corpus bursae gradually enlarging anteriorly, membranous, with four tridentaform signa equally spaced around middle of corpus bursae; enlarged bases of signa projecting externally beyond wall of corpus bursae, with spinose branches projecting internally.
Remarks
For many years John Heath, formerly employed at the Experimental Research Station at Monks Wood in England, pursued research on the family Micropterigidae, resulting in about 20 papers on this group (Emmet 1987[20]). Heath had partially completed a revision of the genus Epimartyria, but this was never published. We had not viewed a copy of this manuscript until our publication was in review. In his manuscript, Heath recognized an additional new species from New Jersey, based on specimens collected at Essex County Park by W. D. Kearfott. Our studies found no morphological justification for this species.
Because this is the first taxonomic revision of Epimartyria, there remain some gaps in our knowledge about their biology which cannot be answered with available material and evidence.
Key to species of Epimartyria
Taxon Treatment
- Davis, D; Landry, J; 2012: A review of the North American genus Epimartyria (Lepidoptera, Micropterigidae) with a discussion of the larval plastron ZooKeys, 183: 37-83. doi
Other References
- ↑ Kearfott W (1903) Checklist of the Lepidoptera of Boreal America. American Entomological Society, Philadelphia, 118–125.
- ↑ Dyar H (1902 [1903]) A list of North American Lepidoptera. United States National Museum Bulletin 52 (19): 1-723.
- ↑ Meyrick E (1912) Lepidoptera Heterocera, Fam. Micropterigidae. Genera Insectorum fascicle 132: 1–9. [1 color plate]
- ↑ 4.0 4.1 Forbes W (1923) The Lepidoptera of New York and neighboring states. Cornell University Agricultural Experiment Station Memoir 68: 1-729.
- ↑ McDunnough J (1939) Check List of the Lepidoptera of Canada and the United States of America, part 2 (Microlepidoptera). Memoirs of the Southern California Academy of Science 2 (2): 1-171.
- ↑ Davis D (1983) Micropterigidae. In: RW Hodges et al. (Eds) Check List of the Lepidoptera of America North of Mexico. EW Classey Ltd and the Wedge Entomological Research Foundation, London, 5–7.
- ↑ Davis D (1987) Micropterigidae, Eriocraniidae. In: FW S (Ed). Immature insects. Volume 1, Kendall/Hunt Publishing, Dubuque, Iowa: 341-343.
- ↑ Kristensen N (1984b) Skeletomuscular anatomy of the male genitalia of Epimartyria (Lepidoptera: Micropterigidae). Entomologica scandinavica 15: 97-112. doi: 10.1163/187631284X00091
- ↑ Nye I, DS F (1991) The Generic Names of Moths of the World. Microlepidoptera. Volume 6(29), Natural History Museum Publications, London, 1–368.
- ↑ Poole R (1996) Diptera, Lepidoptera. In: Poole R Gentili P (Eds) Nomina Insecta Nearctica 3, 1143 pp.
- ↑ 11.0 11.1 11.2 11.3 Hashimoto S (2006) A taxonomic study of the family Micropterigidae (Lepidoptera, Micropterigoidea) of Japan, with the phylogenetic relationships among the Northern Hemisphere genera. Bulletin of the Kitakyushu Museum of Natural History and Human History Series A Natural History 4: 39-109.
- ↑ Kobayashi Y, Suzuki H, Hashimoto H, Gibbs G (2000) Molecular phylogeny of the Japanese and New Zealand Micropterigidae (Lepidoptera) based on mitochondrial gene sequences. Proceedings of the XXI International Congress of Entomology, 2000, Iguazu, Brasil, 956 pp.
- ↑ Gibbs G, Hashimoto S, Kobayashi Y, Lees D, Saigusa T, Sugimoto M, Suzuki H (2004) Molecular phylogeny of Micropterigidae (Lepidoptera). Proceedings of XXII International Congress of Entomology, 2004. Brisbane, Australia. [Abstract only]
- ↑ Hirowatari T, Hashimoto S, Jinbo U, Wang M (2009) Descriptions of two new species of Vietomartyria Hashimoto & Mey (Lepidoptera, Micropterigidae) from South China, with reference to autapomorphies of the genus. Entomological Science 12: 67-73. doi: 10.1111/j.1479-8298.2009.00305.x
- ↑ Faucheux M (1997) Sensory organs on the antennae of Micropterix calthella L. (Lepidoptera, Micropterigidae). Acta Zoologica (Stockholm) 78 (1): 1-8. doi: 10.1111/j.1463-6395.1997.tb01121.x
- ↑ Davis D (1975) A review of the West Indian moths of the family Psychidae with descriptions of new taxa and immature stages. Smithsonian Contributions to Zoology 188: 1-66. doi: 10.5479/si.00810282.188
- ↑ Kristensen N, Simonsen T (2003) Hairs and scales. In: Kristensen N (Ed). Lepidoptera, Moths and butterflies, 2: Morphology, Physiology, and Development. Handbook of Zoology 4(36): 9-22.
- ↑ Philpott A (1925) On an abdominal scent-organ (?) in Sabatinca and other primitive genera of Lepidoptera. Transactions of the entomological Society of London 1924: 457-461.
- ↑ Kristensen N (1984a) The pregenital abdomen of the Zeugloptera (Lepidoptera). Steenstrupia 10(4): 113–136. [Bulletin of the Sugadaira Montane Research Center 11: 101–102]
- ↑ Emmet A (1987) John Heath (1922–1987). Antenna 11 (4): 127-129.
Images
|