Calliscelio
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Chen2017ZooKeys, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Chen2017ZooKeys">{{Citation See also the citation download page at the journal. |
Ordo: Hymenoptera
Familia: Platygastridae
Name
Calliscelio Ashmead – Wikispecies link – Pensoft Profile
- Calliscelio Ashmead, 1893: 209, 218 (original description. Type: Calliscelio laticinctus Ashmead, by monotypy and original designation, keyed); Ashmead 1894[1]: 216 (keyed); Dalla Torre 1898[2]: 501 (catalog of species); Ashmead 1900[3]: 327 (list of species of West Indies); Ashmead 1903[4]: 91 (keyed); Brues 1908[5]: 27, 28, 33 (diagnosis, list of species, keyed); Kieffer 1908[6]: 122 (keyed); Kieffer 1910b[7]: 66 (keyed); Kieffer 1913[8]: 232 (description); Kieffer 1926[9]: 273, 499 (description, keyed, key to species); Muesebeck and Walkley 1956[10]: 338 (citation of type species); Baltazar 1966[11]: 185 (cataloged, catalog of species of the Philippines); Masner 1972[12]: 839 (junior synonym of Calotelea Westwood); Masner 1976[13]: 34, 36, 43 (description; key to Calliscelio Ashmead, Paridris Kieffer, Oethecoctonus Ashmead, and Probaryconus Kieffer; key to Calotelea Westwood and Calliscelio Ashmead); Mani and Sharma 1982[14]: 178 (description); Galloway and Austin 1984[15]: 8, 27, 28 (description, list of species described from Australia, keyed); Kozlov and Kononova 1985[16]: 19 (description, key to species of the Palearctic); Kozlov and Kononova 1990[17]: 19, 173, 183 (description, key to species of the USSR, keyed); Johnson 1992[18]: 355 (catalog of world species); Kononova 1995[19]: 61, 69 (keyed, diagnosis, key to species of Russian Far East); Austin and Field 1997[20]: 20, 68 (structure of ovipositor system, discussion of phylogenetic relationships); Narendran and Ramesh Babu 1990: 2 (key to species of India); Lê 2000[21]: 31, 46 (keyed, description, key to species); Loiácono and Margaría 2002[22]: 557 (catalog of Brazilian species); Mineo 2004[23]: 174 (distribution in Sicily); Rajmohana 2006[24]: 116, 119, 120 (description, keyed, key to species of India); Kononova and Fursov 2007a[25]: 57 (description); Kononova and Fursov 2007b[26]: 98 (description); Kononova and Kozlov 2008[27]: 23, 257, 258 (description, keyed, key to species of Palearctic region); Rajmohana and Peter 2013[28]: 76 (key to species Calliscelio rugosus Rajmohana & Peter and Calliscelio agaliensis Narendran & Ramesh Babu); Talamas and Buffington 2015[29]: 12. (fossil in Dominican amber); Talamas, Johnston-Jordan and Buffington 2016[30]: 413, 416 (description, synonymy). http://zoobank.org/29B1D7E4-1173-4D61-B695-CA755632F5EA http://bioguid.osu.edu/xbiod_concepts/461
- Baryteleia Kiefffer, 1926: 273, 544 (original description. Type: Macroteleia nigriceps Kieffer, by original designation, keyed, key to species); Muesebeck and Walkley 1956[10]: 336 (citation of type species); Masner 1976[13]: 36 (junior synonym of Calliscelio Ashmead). http://zoobank.org/AB0DBC82-18D8-431F-9069-783F4874CEC1 http://bioguid.osu.edu/xbiod_concepts/8406
- Caenoteleia Kieffer, 1926: 266, 550 (original description. Type: Caloteleia elegans Perkins, by monotypy, keyed); Muesebeck and Walkley 1956[10]: 338 (citation of type species); Johnson 1992[18]: 355 (catalog of world species); Masner et al. 2009[31]: 60 (junior synonym of Calliscelio Ashmead, discussion of status). http://zoobank.org/5FEFDDD1-26AD-40D7-A498-BB4B5DF03630 http://bioguid.osu.edu/xbiod_concepts/460
- Ceratoteleia Kieffer, 1908: 121 (original description. Type Caloteleia grenadensis Ashmead, designated by Kieffer (1926)[9], keyed); Kieffer 1910b[7]: 65, 66, 88 (description, list of species, keyed); Dodd 1913a[32]: 131, 144 (key to species of Australia); Dodd 1913b[33]: 176 (comparison with Macroteleia Westwood); Kieffer 1913[8]: 222 (description); Kieffer 1913[8]: 232 (description); Kieffer 1914a[34]: 315 (description, key to species of Europe and Algeria); Kieffer 1926[9]: 273, 500 (description, keyed, key to species, designation of type species); Nixon 1931[35]: 356 (keyed, key to species of Africa); Nixon 1933[36]: 292 (keyed); Brues 1940[37]: 82 (key to species of Baltic amber); Maneval 1940[38]: 114 (keyed); Risbec 1950[39]: 603 (key to species of Ethiopian region); Muesebeck and Walkley 1951[40]: 705 (catalog of species of U.S. and Canada); Muesebeck and Walkley 1956[10]: 341 (citation of type species); Masner 1976[13]: 36 (junior synonym of Calliscelio Ashmead). http://zoobank.org/70CBF446-7CD7-4211-BF0C-F401DAE92DDA http://bioguid.osu.edu/xbiod_concepts/8400
- Crama Galloway, 1984: 7, 8, 28 (original description. Type: Baryconus albicoxa Dodd, by original designation, key to Australian species, keyed); Johnson 1992[18]: 364 (catalog of world species); Talamas, Johnston-Jordan and Buffington 2016[30]: 413, 417 (junior synonym of Calliscelio Ashmead). http://zoobank.org/10BDF90E-0D3E-491E-98B2-8B39BB9D5871 http://bioguid.osu.edu/xbiod_concepts/466
- Glyptoteleia Kieffer, 1926: 272, 487 (original description. Type: Baryconus bisulcatus Kieffer, by monotypy, keyed); Muesebeck and Walkley 1956[10]: 356 (citation of type species); Szabó 1962[41]: 241 (diagnosis); Masner 1976[13]: 36 (junior synonym of Calliscelio Ashmead); De Santis 1980[42]: 312 (catalog of species of Brazil). http://zoobank.org/FFA0AF0B-A126-4A36-8DB4-E25910A88C3D http://bioguid.osu.edu/xbiod_concepts/8405
- Lispoteleia Galloway, 1984: 7, 9, 35 (original description. Type: Lispoteleia collina Galloway, by original designation, key to species of Australia, keyed); Johnson 1992[18]: 421 (catalog of world species); Austin and Field 1997[20]: 22, 68 (structure of ovipositor system, discussion of phylogenetic relationships); Talamas, Johnston-Jordan and Buffington 2016[30]: 413, 417 (junior synonym of Calliscelio Ashmead). http://zoobank.org/A458DE09-DAFA-424E-B60E-1A831B19D01E http://bioguid.osu.edu/xbiod_concepts/503
- Mesoteleia Kieffer, 1917: 51 (original description. Type: Mesoteleia pallida Kieffer, by monotypy and original designation); Kieffer 1926[9]: 271, 441 (description, keyed); Muesebeck and Walkley 1956[10]: 369 (citation of type species); Baltazar 1966[11]: 182 (cataloged, catalog of species of the Philippines); Masner 1976[13]: 36 (junior synonym of Calliscelio Ashmead). http://zoobank.org/B6CD6365-672B-4E49-B3AE-6AAFA9C42DA6 http://bioguid.osu.edu/xbiod_concepts/8404
- Prosanteris Kieffer, 1908: 121, 136 (original description. Type: Anteris nigriceps Ashmead, designated by Kieffer (1910b)[7], keyed); Kieffer 1910b[7]: 65, 87 (description, key to subgenera, list of species, keyed); Kieffer 1913[8]: 232 (description); Kieffer 1926[9]: 272, 437 (description, keyed, key to species); Muesebeck and Walkley 1951[40]: 704 (catalog of species of U.S. and Canada); Muesebeck and Walkley 1956[10]: 391 (citation of type species); Muesebeck 1958: 93 (junior synonym of Ceratoteleia Kieffer). http://zoobank.org/31DB13FB-2699-4E1D-A023-1BA21C1B9503 http://bioguid.osu.edu/xbiod_concepts/8401
- Uroscelio Kieffer, 1914: 291 (original description. Type: Uroscelio luteipes Kieffer, by monotypy and original designation); Kieffer 1926[9]: 268, 409 (description, keyed); Muesebeck and Walkley 1956[10]: 408 (citation of type species); Baltazar 1966[11]: 180 (cataloged, catalog of species of the Philippines); Masner 1976[13]: 36 (junior synonym of Calliscelio Ashmead). http://zoobank.org/91A2A4C7-7D11-4723-8C53-02DEA8A0213B http://bioguid.osu.edu/xbiod_concepts/8403
- Xentor Masner & Johnson, 2007: 12, 14 (original description. Type: Xentor schlingeri Masner & Johnson, by original designation, key to species); Talamas, Johnston-Jordan and Buffington 2016[30]: 416 (junior synonym of Calliscelio Ashmead). http://zoobank.org/1578C9FB-4A24-42D6-922D-05CDA2626906 http://bioguid.osu.edu/xbiod_concepts/211604
- Yunkara Galloway, 1984: 9, 33 (original description. Type: Yunkara inornata Galloway, by monotypy and original designation, keyed); Johnson 1992[18]: 510 (catalog of world species); Talamas, Johnston-Jordan and Buffington 2016[30]: 413, 418 (junior synonym of Calliscelio Ashmead). http://zoobank.org/4FBE9CB9-3B71-4DFB-BCE0-781664A31929 http://bioguid.osu.edu/xbiod_concepts/578
Description
(based on New World species). Length: 1.27–3.88 mm; body moderately to markedly elongate, robust.
Head. Head shape in dorsal view: transverse. Hyperoccipital carina: absent; present. Occipital carina: present, complete medially; present laterally, broadly interrupted medially; completely absent. Occipital carina sculpture: crenulate; unsculptured. OOL: lateral ocellus nearly contiguous with inner orbits, OOL < 0.5 OD; lateral ocellus contiguous with inner orbit. Upper frons: convex, without frontal shelf. Scrobe shape: frons broadly convex, without distinct scrobe. Frons sculpture: scrobe largely smooth, otherwise granulate or variably punctate. Submedian carina: absent. Orbital carina: absent. Inner orbits: diverging ventrally. IOS/EH: IOS distinctly less than EH; IOS slightly greater than EH. Interantennal process: short, often excavate medially. Central keel: present; absent. Torulus opening: laterally on interantennal process. Lower frons striae: absent. Malar sulcus: present. Compound eye size: of normal proportions, not significantly reduced. Compound eye setation: glabrous; sparsely setose; densely setose. Gena: broad, convex, distinctly produced behind eye. Clypeus shape: narrow, slightly convex medially, lateral corner not produced. Apical margin of clypeus: straight. Anteclypeus: absent. Postclypeus: absent. Labrum: not visible. Mandible shape: moderate. Mandibular teeth: apex with 3, acute, subequal teeth. Arrangement of mandibular teeth: transverse. Number of maxillary palpomeres: 4. Shape of maxillary palpomeres: cylindrical. Number of labial palpomeres: 2.
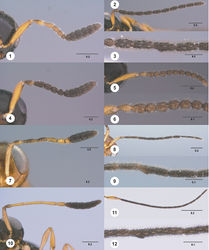
Antenna. Number of antennomeres in female: 12. Number of antennomeres in male: 12. Insertion of radicle into A1: parallel to longitudinal axis of A1. Shape of A1: more or less cylindrical, not flattened. Length of A3 of female: distinctly longer than A2. Number of clavomeres in female antenna: 6. Claval formula of female antenna: A12–A7/1-2-2-2-2-1. Arrangement of doubled multiporous plate sensilla on female clava: in longitudinal pairs. Tyloid distribution on male antenna: A5 only. Shape of male flagellum: filiform.
Mesosoma. Mesosoma shape in dorsal view: longer than wide. Mesosoma shape in lateral view: longer than high. Medial portion of transverse pronotal carina: weakly indicated laterally; absent. Posterior apex of pronotum in dorsal view: straight, bifid apically to articulate with tegula. Vertical epomial carina: absent. Dorsal epomial carina (lateral portion of transverse pronotal carina of Vilhelmsen et al. 2010[43]): present. Anterior face of pronotum: oblique, visible dorsally, short. Lateral face of pronotum: weakly concave below position of dorsal epomial carina. Netrion: present. Netrion shape: narrow to moderately wide, open ventrally. Anterior portion of mesoscutum: vertical, flexed ventrally to meet pronotum. Mesoscutum shape: semielliptical, excavate at base of wings. Skaphion: absent. Notauli: present, percurrent; present, abbreviated. Parapsidal lines: absent. Admedial lines: absent. Transscutal articulation: well-developed, narrow. Shape of mesoscutellum: quadrate to trapezoidal. Armature of mesoscutellum: absent. Surface of mesoscutellum: convex throughout. Median longitudinal furrow on mesoscutellum: absent. Shape of axillula: small, dorsal margin sinuate. Metascutellum: clearly differentiated. Metascutellar armature: absent. Metascutellar setation: glabrous. Metapostnotum: not defined externally. Extent of metasomal depression of propodeum: percurrent, extending anteriorly to anterior margin of propodeum. Lateral propodeal projection: absent. Mesopleural carina: present, extending at least to sternaulus; absent or strongly abbreviated, present only near mid coxa. Mesal course of acetabular carina: projecting as small spur anteriorly, not separating fore coxae. Mesopleural pit: present. Sternaulus: absent. Posterodorsal corner of mesopleuron: rounded anteriorly.
Legs. Number of mid tibial spurs: 1. Number of hind tibial spurs: 1. Dorsal surface of hind coxa: smooth; transversely rugose. Hind tibia shape: cylindrical, ecarinate. Trochantellus: indicated by transverse sulcus on femur.
Wings. Wing development of female: macropterous. Wing development of male: macropterous. Tubular veins in fore wing: present. Bulla of fore wing R: absent. Extent of marginal venation of fore wing: distinct marginal or postmarginal veins developed. Origin of r-rs in fore wing: arising from marginal vein along costal margin. Development of basal vein (Rs+M) in fore wing: spectral; nebulous, strongly pigmented; absent. Development of R in hind wing: elongate, extending to costal margin.
Metasoma. Number of external terga in female: 6. Number of external sterna in female: 6. Number of external terga in male: 7. Number of external sterna in male: 7. Shape of metasoma: lanceolate. Laterotergites: present, narrow. Laterosternites: present. T1 of female: more or less evenly convex; produced medially into cylindrical or elliptical horn housing ovipositor. Relative size of metasomal segments: T2–T4 largest, subequal in size. Terga with basal crenulae: T2. Sublateral carinae on tergites: absent. Median longitudinal carina on metasomal terga: absent. Shape of female T6: flattened. Shape of posterior margin of male T7: rounded. Anterior margin of S1: not produced anteriorly, concave. Distribution of felt fields: absent. Ovipositor type: Scelio-type (Austin and Field 1997[20]).
Diagnosis
Calliscelio may be distinguished from other genera of the subfamily by the combination of the following characters: eyes glabrous in many species but in some with short hairs or even densely hairy; skaphion never developed; metanotum medially produced into a transverse plate or lamella, neither spinose nor toothed laterally; propodeum usually unarmed, often excavate to contain T1 horn, only in a few species with posterolateral corner acute; T6 in females often elongate, sword-like, depressed dorsoventrally. Calliscelio is most similar to Holoteleia Kieffer and Probaryconus Kieffer in the tribe Calliscelionini and Calotelea in Psilanteridini in body shape and some external characters. The following key is used to separate these genera with the fewest characters possible.
Key to separate Calliscelio, Calotelea, Holoteleia and Probaryconus
New World species of Calliscelio Ashmead
Calliscelio absconditum Chen & Johnson, sp. n.
Calliscelio absum Chen & Johnson, sp. n.
Calliscelio alcoa Chen & Masner, sp. n.
Calliscelio amadoi Chen & Johnson, sp. n.
Calliscelio armila Chen & Masner, sp. n.
Calliscelio bidens Chen & Masner, sp. n.
Calliscelio bisulcatus (Kieffer, 1910)
Calliscelio brachys Chen & Johnson, sp. n.
Calliscelio brevinotaulus Chen & Johnson, sp. n.
Calliscelio brevitas Chen & Johnson, sp. n.
Calliscelio carinigena Chen & Johnson, sp. n.
Calliscelio crater Chen & Johnson, sp. n.
Calliscelio crena Chen & Johnson, sp. n.
Calliscelio eboris Chen & Johnson, sp. n.
Calliscelio elegans (Perkins)
Calotelea tanugatra Narendran
Calliscelio extenuatus Chen & Johnson, sp. n.
Calliscelio flavicauda Chen & Johnson, sp. n.
Calliscelio foveolatus Chen & Johnson, sp. n.
Calliscelio gatineau Chen & Johnson, sp. n.
Calliscelio glaber Chen & Masner, sp. n.
Calliscelio granulatus Chen & Masner, sp. n.
Calliscelio laticinctus Ashmead, 1893
Calliscelio latifrons Chen & Johnson, sp. n.
Calliscelio levis Chen & Johnson, sp. n.
Calliscelio longius Chen & Johnson, sp. n.
Calliscelio magnificus Chen & Masner, sp. n.
Calliscelio migma Chen & Johnson, sp. n.
Calliscelio minutia Chen & Johnson, sp. n.
Calliscelio paraglaber Chen & Johnson, sp. n.
Calliscelio pararemigio Chen & Masner, sp. n.
Calliscelio prolixus Chen & Johnson, sp. n.
Calliscelio punctatifrons Chen & Johnson, sp. n.
Calliscelio remigio Chen & Masner, sp. n.
Calliscelio rubriclavus (Ashmead, 1887), comb. n.
Anteris nigriceps Ashmead, 1893, syn. n.
Caloteleia Marlattii Ashmead, 1893, syn. n.
Caloteleia grenadensis Ashmead, 1896, syn. n.
Macroteleia ruskini Girault, 1920, syn. n.
Calliscelio ruga Chen & Johnson, sp. n.
Calliscelio rugicoxa Chen & Masner, sp. n.
Calliscelio sfina Chen & Johnson, sp. n.
Calliscelio storea Chen & Johnson, sp. n.
Calliscelio suni Chen & Johnson, sp. n.
Calliscelio telum Chen & Johnson, sp. n.
Calliscelio torqueo Chen & Johnson, sp. n.
Calliscelio virga Chen & Johnson, sp. n.
Key to females of Calliscelio of the New World
Key to males
(unknown for Calliscelio amadoi, Calliscelio bidens, Calliscelio brevitas, Calliscelio foveolatus, Calliscelio gatineau, Calliscelio levis, Calliscelio prolixus, Calliscelio rugicoxa, Calliscelio ruga and Calliscelio storea)
Taxon Treatment
- Chen, H; Masner, L; Johnson, N; 2017: New World species of the genus Calliscelio Ashmead (Hymenoptera, Platygastridae, Scelioninae) ZooKeys, (648): 1-136. doi
Images
|
Other References
- ↑ Ashmead W (1894) Report on the parasitic Cynipidae, part of the Braconidae, the Ichneumonidae, the Proctotrypidae, and part of the Chalcidinae. Part III. Zoological Journal of the Linnean Society of London 25: 188–254. https://doi.org/10.5281/zenodo.23423
- ↑ Dalla Torre C (1898) Catalogus hymenopterorum hucusque descriptiorum systematicus et synonymicus. Vol. V: Chalcididae et Proctotrupidae. Sumptibus Guilelmi Engelmann, Lipsiae, 598 pp. https://doi.org/10.5962/bhl.5i5le.10348
- ↑ Ashmead W (1900) Report upon the aculeate Hymenoptera of the islands of St. Vincent and Grenada, with additions to the parasitic Hymenoptera and a list of the described Hymenoptera of the West Indies. Transactions of the Royal Entomological Society of London 1900: 207–367. https://doi.org/10.5281/zenodo.23429
- ↑ Ashmead W (1903) Classification of the pointed-tailed wasps, or the superfamily Proctotrypoidea – III. Journal of the New York Entomological Society 11: 86–99. https://doi.org/10.5281/zenodo.23553
- ↑ Brues C (1908) Hymenoptera. Fam. Scelionidae. Genera Insectorum 80: 1–59. https://doi.org/10.5281/zenodo.23624
- ↑ Kieffer J (1908) Revision des Scelionidae (Hyménoptères). Annales de la Société Scientifique de Bruxelles. Mémoires 32: 111–250. https://doi.org/10.5281/zenodo.23670
- ↑ 7.0 7.1 7.2 7.3 Kieffer J (1910b) Hymenoptera. Fam. Scelionidae. Addenda et corrigenda. Genera Insectorum 80: 61–112. https://doi.org/10.5281/zenodo.23671
- ↑ 8.0 8.1 8.2 8.3 Kieffer J (1913) Proctotrypidae (3e partie). Species des Hyménoptères d’Europe et d’Algerie 11: 161–304. https://doi.org/10.5281/zenodo.23682
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Kieffer J (1926) Scelionidae. Das Tierreich. Vol. 48. Walter de Gruyter & Co., Berlin, 885 pp.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 Muesebeck C, Walkley L (1956) Type species of the genera and subgenera of parasitic wasps comprising the superfamily Proctotrupoidea (order Hymenoptera). Proceedings of the U.S. National Museum 105: 319–419. https://doi.org/10.5479/si.00963801.3359.319
- ↑ 11.0 11.1 11.2 Baltazar C (1966) A catalogue of Philippine Hymenoptera (with a bibliography, 1758–1963). Pacific Insects Monographs 8: 1–488. https://doi.org/10.5281/zenodo.23590
- ↑ Masner L (1972) The classification and interrelationships of Thoronini (Hymenoptera: Proctotrupoidea, Scelionidae). The Canadian Entomologist 104: 833–849. https://doi.org/10.4039/Ent104833-6
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Masner L (1976) Revisionary notes and keys to world genera of Scelionidae (Hymenoptera: Proctotrupoidea). Memoirs of the Entomological Society of Canada 97: 1–87. https://doi.org/10.4039/entm10897fv
- ↑ Mani M, Sharma S (1982) Proctotrupoidea (Hymenoptera) from India. A review. Oriental Insects 16: 135–258. https://doi.org/10.5281/zenodo.23680
- ↑ Galloway I, Austin A (1984) Revision of the Scelioninae (Hymenoptera: Scelionidae) in Australia. Australian Journal of Zoology Supplementary Series 99: 1–138. https://doi.org/10.1071/AJZS099
- ↑ Kozlov M, Kononova S (1985) [A review of the genera Triteleia and Calliscelio (Proctotrupoidea, Scelionidae).] Vestnik Zoologii 1985(4): 15–24. https://doi.org/10.5281/zenodo.23774
- ↑ Kozlov M, Kononova S (1990) [[[Scelioninae|Scelioninae]] of the Fauna of the USSR (Hymenoptera, Scelionidae, Scelioninae).] Nauka, Leningrad, 344 pp.
- ↑ 18.0 18.1 18.2 18.3 18.4 Johnson N (1992) Catalog of world Proctotrupoidea excluding Platygastridae. Memoirs of the American Entomological Institute 51: 1–825. https://doi.org/10.5281/zenodo.23657
- ↑ Kononova S (1995) [Fam. Scelionidae.] Pages 57–121 in Lehr P. [Key to insects of Russian Far East in six volume. vol. 4. Neuropteroidea, Mecoptera, Hymenoptera. Part 2. Hymenoptera]. Dal’nauka, Vladivostok, 600 pp.
- ↑ 20.0 20.1 20.2 Austin A, Field S (1997) The ovipositor system of scelionid and platygastrid wasps (Hymenoptera: Platygastroidea): comparative morphology and phylogenetic implications. Invertebrate Taxonomy 11: 1–87. https://doi.org/10.1071/IT95048
- ↑ Lê X (2000) Egg-parasites of family Scelionidae (Hymenoptera). Fauna of Vietnam, vol. 3. Science and Technics Publishing House, Hanoi, 386 pp.
- ↑ Loiácono M, Margaría C (2002) Systematics, morphology and physiology. Ceraphronoidea, Platygastroidea and Proctotrupoidea from Brazil (Hymenoptera). Neotropical Entomology 31(4): 551–560. https://doi.org/10.1590/S1519-566X2002000400007
- ↑ Mineo G (2004) Description of new taxa, both in Scelioninae and Telenominae (Hymenoptera Scelionidae). Bollettino di Zoologia Agraria e Bachicoltura 36(2): 173–188.
- ↑ Rajmohana K (2006) Studies on Proctotrupoidea and Platygastroidea (Hymenoptera: Insecta) of Kerala. Memoirs of the Zoological Survey of India 21(1): 1–153.
- ↑ Kononova S, Fursov V (2007a) [A review of the genera Calotelea, Calliscelio, and Oxyscelio (Scelioninae, Scelionidae, Proctotrupoidea) from the Palaearctic fauna.] Zoologicheskii Zhurnal 86: 52–65.
- ↑ Kononova S, Fursov V (2007b) A review of the genera Calotelea, Calliscelio, and Oxyscelio (Scelioninae, Scelionidae, Proctotrupoidea) from the Palaearctic fauna. Entomological Review 87: 92–105. https://doi.org/10.1134/S0013873807010101
- ↑ Kononova S, Kozlov M (2008) [Scelionids of the Palearctic (Hymenoptera, Scelionidae). Subfamily Scelioninae. Tovarishchestvo Nauchnykh Izdanii KMK, Saint Petersburg, 489 pp.
- ↑ Rajmohana K, Peter A (2013) A new species of Calliscelio Ashmead (Platygastridae: Hymenoptera: Insecta) from India. Records of the Zoological Survey of India 112(1): 75–79.
- ↑ Talamas E, Buffington M (2015) Fossil Platygastroidea in the National Museum of Natural History, Smithsonian Institution. Journal of Hymenoptera Research 47: 1–52. https://doi.org/10.3897/JHR.47.5730
- ↑ 30.0 30.1 30.2 30.3 30.4 Talamas E, Johnston-Jordan D, Buffington M (2016) Calliscelio Ashmead expands (Hymenoptera: Scelionidae). Proceedings of the Entomological Society of Washington 118: 404–423. https://doi.org/10.4289/0013-8797.118.3.404
- ↑ Masner L, Johnson F, Musetti L (2009) Calliscelio elegans (Perkins), a tramp species, and a review of the status of the genus Caenoteleia Kieffer (Hymenoptera: Platygastridae). Zootaxa 2237: 59–66.
- ↑ Dodd A (1913a) Australian Hymenoptera Proctotrypoidea. No. 1. Transactions of the Royal Society of South Australia 37: 130–181. https://doi.org/10.5281/zenodo.23698
- ↑ Dodd A (1913b) Some new parasitic Hymenoptera from Australia. Archiv für Naturgeschichte 79(6): 164–182. https://doi.org/10.5281/zenodo.23772
- ↑ Kieffer J (1914a) Proctotrypidae (3e partie). Species des Hymenopteres d’Europe et d’Algerie 11: 305–448. https://doi.org/10.5281/zenodo.23689
- ↑ Nixon G (1931) On some new South African Proctotrupoidea (Hymenoptera). Eos 7: 355–382. https://doi.org/10.5281/zenodo.23773
- ↑ Nixon G (1933) A further contribution to the study of South Africa Scelionidae (Insecta, Hymenoptera, Proctotrupoidea). Annals and Magazine of Natural History (10)12: 288–563. https://doi.org/10.5281/zenodo.23692
- ↑ Brues C (1940) Fossil parasitic Hymenoptera of the family Scelionidae from Baltic amber. Proceedings of the American Academy of Arts & Sciences 74: 69–90. https://doi.org/10.2307/20023360
- ↑ Maneval H (1940) Fam. XVII. Proctotrypides. Pages 93–118 in Perrier R. La Faune de la France en tableaux synoptiques illustrés. Tome VII. Hyménoptères par Lucien Berland avec la collaboration de MM. Raymond Benoit, Francis Bernard, Henri Maneval, Paris, 213 pp.
- ↑ Risbec J (1950) Contribution à l’étude des Proctotrupidae (Serphiidae). Pages 511–639 in Risbec J. Travaux du Laboratoire d’Entomologie du Secteur Soudanis de Recherches Agronomiques. Gouvernement Général de l’Afrique Occidentale Française, Paris, 639 pp.
- ↑ 40.0 40.1 Muesebeck C, Walkley L (1951) Superfamily Proctotrupoidea. Pages 655–718 in Muesebeck C Krombein K Townes H. Hymenoptera of America north of Mexico – Synoptic Catalog. U.S. Dept. Agriculture Monograph No. 2, 1420 pp. https://doi.org/10.5962/bhl.title.65057
- ↑ Szabó J (1962) Untersuchungen an palaearktischen Proctotrupiden. I–IV. (Hymenoptera). Folia Entomologica Hungarica 15: 221–243. https://doi.org/10.5281/zenodo.23754
- ↑ De Santis L (1980) Catálogo de los himenópteros brasileños de la serie Parasitica incluyendo Bethyloidea. Editora da Universidade Federal do Paraná, Curitiba, Brazil, 395 pp.
- ↑ Vilhelmsen L, Mikó I, Krogmann L (2010) Beyond the wasp-waist: structural diversity and phylogenetic significance of the mesosoma in apocritan wasps (Insects: Hymenoptera). Zoological Journal of the Linnean Society 159: 22–194. https://doi.org/10.1111/j.1096-3642.2009.00576.x