Blythophryne
Contents
- 1 Taxonavigation
- 2 Name
- 3 Type species
- 4 Content
- 5 Type material
- 6 Etymology
- 7 Diagnosis
- 8 Description of the holotype
- 9 Colouration in life
- 10 Colouration in alcohol
- 11 Osteology
- 12 Morphological variations
- 13 Description of calls
- 14 Distribution
- 15 Vernacular name
- 16 Ecological notes
- 17 Conservation status
- 18 Notes on larval development
- 19 Description of Tadpole
- 20 Colour
- 21 Morphological comparisons
- 22 Molecular phylogeny
- 23 Original Description
- 24 Images
- 25 Other References
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Chandramouli2016ZooKeys, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Chandramouli2016ZooKeys">{{Citation See also the citation download page at the journal. |
Name
Blythophryne Chandramouli & Vasudevan & Harikrishnan & Dutta & Janani & Sharma & Das & Aggarwal, 2016 gen. n. – Wikispecies link – ZooBank link – Pensoft Profile
Type species
Blythophryne beryet gen. et sp. n. by monotypy (Fig. 1, Table 4).
| ZSI A-12521 | ZSI A-12524 | ZSI A-12522 | ZSI A-12523 | ZSI A-12526 | ZSI A-12529 | ZSI A-12527 | ZSI A-12530 | ZSI A-12528 | ZSI A-12525 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ♀ | ♀(g) | ♂ | ♀(g) | ♂ | ♂ | ♀ | ♂ | ♂ | ♂ |
| SVL | 27.4 | 25.5 | 25.5 | 25.2 | 24.5 | 23.0 | 22.7 | 22.3 | 22.2 | 21.8 |
| AG | 10.6 | 9.2 | 9.8 | 12.5 | 8.0 | 7.3 | 8.0 | 6.7 | 6.5 | 8.5 |
| HL | 7.7 | 7.5 | 8.2 | 6.9 | 7.5 | 7.9 | 7.5 | 7.6 | 7.6 | 7.1 |
| HW | 7.9 | 7.6 | 8.1 | 6.8 | 8.0 | 7.8 | 7.6 | 7.7 | 7.4 | 7.2 |
| HD | 4.3 | 3.5 | 3.9 | 3.4 | 3.9 | 3.4 | 3.2 | 3.2 | 3.0 | 3.2 |
| BW | 9.9 | 10.3 | 9.1 | 11.8 | 8.3 | 7.1 | 6.1 | 7.5 | 6.3 | 9.8 |
| EN | 2.2 | 2.3 | 1.7 | 1.9 | 1.9 | 2.3 | 2.1 | 2.0 | 2.1 | 2.2 |
| ES | 3.4 | 3.4 | 3.3 | 3.1 | 3.5 | 3.3 | 3.2 | 3.2 | 3.3 | 3.1 |
| ETY | 0.5 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.6 | 0.6 | 0.5 |
| UEW | 1.9 | 1.5 | 2.0 | 1.8 | 1.8 | 1.7 | 1.7 | 1.5 | 1.9 | 1.8 |
| IO | 3.8 | 3.8 | 3.5 | 3.4 | 3.5 | 3.8 | 3.4 | 3.5 | 3.6 | 3.4 |
| IN | 2.2 | 2.1 | 2.2 | 2.2 | 1.8 | 2.1 | 2.1 | 1.9 | 2.0 | 1.9 |
| TYH | 1.6 | 1.6 | 1.9 | 1.6 | 1.6 | 1.4 | 1.5 | 1.4 | 1.8 | 1.5 |
| TYV | 1.8 | 1.7 | 1.9 | 1.6 | 1.6 | 1.6 | 1.6 | 1.5 | 1.8 | 1.5 |
| UAL | 5.1 | 4.7 | 4.4 | 5.2 | 4.3 | 4.3 | 4.3 | 4.6 | 4.3 | 4.1 |
| LAL | 5.8 | 5.4 | 5.6 | 5.5 | 5.6 | 5.5 | 5.4 | 5.3 | 5.4 | 5.3 |
| PAL | 6.2 | 5.7 | 6.2 | 6.8 | 5.8 | 5.9 | 5.9 | 5.8 | 5.9 | 6.1 |
| FEL | 9.2 | 7.5 | 7.7 | 7.2 | 9.3 | 8.2 | 9.5 | 8.2 | 8.6 | 8.5 |
| TBL | 10.6 | 8.0 | 9.4 | 8.4 | 9.1 | 7.9 | 9.0 | 8.1 | 8.3 | 8.5 |
| FOL | 9.6 | 9.7 | 9.4 | 8.3 | 9.4 | 8.0 | 9.2 | 8.3 | 8.7 | 8.3 |
| ED | 2.8 | 2.5 | 2.4 | 2.6 | 2.5 | 2.1 | 1.9 | 2.4 | 2.1 | 2.3 |
| DL fold | 13.3 | 12.2 | 11.3 | 11.9 | 12.0 | 12.3 | 11.7 | 12.0 | 11.4 | 11.9 |
| PL | 6.1 | 5.9 | 6.5 | 6.0 | 4.5 | 3.7 | 4.0 | 5.9 | 3.2 | 3.9 |
| PW | 1.4 | 1.4 | 1.3 | 1.6 | 1.0 | 1.0 | 0.9 | 1.3 | 0.9 | 0.9 |
| f1 | 1.8 | 1.2 | 1.1 | 1.5 | 1.6 | 1.3 | 2.0 | 1.2 | 1.6 | 0.9 |
| f2 | 1.9 | 1.4 | 1.6 | 1.7 | 2.2 | 1.8 | 2.2 | 1.4 | 1.9 | 1.6 |
| f3 | 3.1 | 3.0 | 2.9 | 2.8 | 2.8 | 2.6 | 2.8 | 2.9 | 2.4 | 2.9 |
| f4 | 2.2 | 1.9 | 1.8 | 2.1 | 2.2 | 1.9 | 2.1 | 1.8 | 1.6 | 1.8 |
| t1 | 1.1 | 1.1 | 1.2 | 1.1 | 1.3 | 1.0 | 1.3 | 1.2 | 1.0 | 1.1 |
| t2 | 1.4 | 1.7 | 1.4 | 1.4 | 1.7 | 1.4 | 1.5 | 1.1 | 1.5 | 1.5 |
| t3 | 2.6 | 2.0 | 2.0 | 2.7 | 2.3 | 2.1 | 2.6 | 2.1 | 2.2 | 1.9 |
| t4 | 4.7 | 4.1 | 3.9 | 4.9 | 4.4 | 2.9 | 4.6 | 3.7 | 3.0 | 4.0 |
| t5 | 3.0 | 2.1 | 2.3 | 2.6 | 2.5 | 2.1 | 2.5 | 2.1 | 2.0 | 1.9 |
Content
A single species is currently known.
Type material
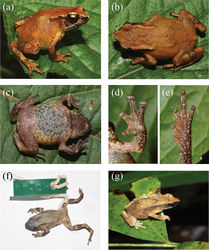
Holotype ♀ : ZSI_A-12521(Fig. 1), (SVL 27.4 mm) leg. S. R. Chandramouli and S. Harikrishnan on 12 December 2010 near Mt. Harriet National Park (ca. 11°42'N, 92°44'E, 175 m asl.) within evergreen forests at ca. 2130 hours. Paratypes (paratopotypes): ZSI_A-12522 to ZSI_A-12530 (three ♀ and six ♂; Fig. 1g); leg. S. R. Chandramouli and S. Harikrishnan during 22 - 25 June 2010 from the above location but at an altitude range of ~150–330 m asl. Other paratypes (larvae): seven tadpoles (WII-115) collected from a phytotelm on Rutland Island. Referred material: WII-113, an adult topotype with six toes on both the feet.
Etymology
The generic name is a patronym, coined in appreciation of Edward Blyth (1810–1873), the first curator of the Asiatic Society of Bengal, who initiated herpetological studies in the Andaman and Nicobar Islands, through his phenomenal, pioneering paper “Notes on the fauna of the Nicobar islands” (Blyth 1846). Das (1999)[1] remarked, “Blyth is to be credited for the description of a large number of species from the Andaman and Nicobar Islands that are still valid. Blyth (1846) wrote the first account on the vertebrate fauna of these islands, and in 1863, compiled the first check-list”. Further details of Edward Blyth and his contributions to studies on Indian natural history are in Das (2004)[2] and Sridharan (2013). The specific epithet ‘beryet’ (in Great Andamanese language; http://www.andamanese.net/Great_Andamanese_Lexicon_English.pdf) refers to ‘small frog’. We believe that the Great Andamanese knew of the existence of this small arboreal anuran that is here described as new species to science. We hope the name given here will also raise awareness about the dwindling, indigenous tribal populations in the Andamans, their culture and extinction of their tribal languages.
Diagnosis
This currently monotypic genus and species is diagnosed by the following suite of external morphological and osteological characters: small adult size (mean SVL 24.0 mm; range 21.8–27.4 mm); distinct tympanum, slightly smaller than eye; absence of cephalic ridges; absence of vomerine teeth; presence of a single, median, external vocal sac in males; presence of elongated pair of parotoid glands; absence of enlarged, keratinised tubercles on dorsum; presence of well developed, sheath-like webbing on fingers and on toes; digit tips dilated to discs, lacking circum-marginal grooves; presence of six presacral vertebrae; urostyle lacking lateral dilations; absence of omosternum and presence of arciferal pectoral girdle. Mature ova small (0.62 mm mean diameter), yolky and unpigmented; tadpoles lacking keratodont.
Description of the holotype
A small bufonid (mean SVL 24.2 ± 0.6 mm), with depressed, moderately robust (AG:BW 1.0) habitus (Fig. 1a–c). Head almost as long as broad (HL:HW 0.97), devoid of cephalic ridges, with a single, median internal vocal sac in males. Snout obtusely pointed in dorsal view, projecting beyond mandibles; nostrils oriented laterally, situated on lateral fold closer to tip of snout than to eye (EN:ES 0.7), loreal region mildly concave, canthal ridge well defined between nostril and the eye, distance between orbit and nostril greater than internarial distance (IN:EN 0.96), upper eyelid rough, densely covered with minute warts, eyes large (ED:HL 0.4), about twice length of tympanum (TYH:ED 0.6), separated from each other by twice internarial distance (IN:IO 0.6), and over twice width of upper eyelid (IO:UEW 1.9), pineal ocellus absent; vomerine teeth absent, tongue elongate, slender and oval, free posteriorly, not bifid, lacking lingual papilla; dorsolateral fold conspicuous, almost up to 48% SVL, beyond which it becomes indistinct and disappears; parotoid glands slender and elongate (PL:PW 4.3), as well-defined postorbital ridge. Limbs slender, upper arm short, 18.7% of SVL, lower arm longer than the upper arm (21% SVL), fingers basally webbed, webbing between Fingers II and III not exceeding penultimate subarticular tubercle (webbing formula I0-1II1-2III2-1IV; Fig. 1d); an enlarged, prominent outer metacarpal tubercle at palmar base (subequal to disc on Finger I), nuptial pad absent , subarticular tubercles prominent on fingers and toes, finger tips dilated to discs lacking circummarginal grooves that are much broader than long, and are less discernible in the first and second fingers; relative length of fingers 3 > 4 > 2 > 1; thigh 33.7% SVL, subequal to shank (38.6% SVL); toes partially webbed, webbing between Toes III and IV extending to penultimate subarticular tubercle (webbing formula I0-1II0-1III1-2IV2½-½V; Fig. 1e); tarsal ridge absent, inner meta-tarsal tubercle larger than outer. Relative length of toes 4 > 5 > 3 > 2 > 1. Skin rough dorsally and granular ventrally; lower abdomen with free, loose skin flap. Tubercles or granules absent on dorsum, scattered over venter, under surface of thighs less granular; throat and limb-insertions with dense granules, tibia with enlarged granular tubercles.
Colouration in life
Dorsum reddish-brown, with two feeble dark brown inverted ‘V’ shaped markings which fail to reach flanks, interorbital band indistinct, canthus dark chocolate brown, colour extending a little beyond tympanum, subequal to half-length of parotoid gland; forearm and hind limbs barred, one each on thigh, shank and tarsus. Venter heavily speckled with dark brown spots, throat dark brown, lower lip spotted with white and brown, pupil large, horizontally elliptical.
Colouration in alcohol
Dorsum drab brown with indistinct ‘inverted-V’ shaped pattern, darker bands on limbs, venter cream, with black mottled pattern, throat black throughout (Fig. 1f).
Osteology
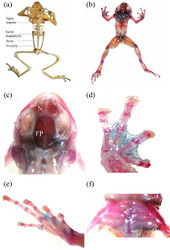
(based on paratype ZSI_A12527). Axial and appendicular skeleton composed primarily of bony elements; cartilaginous elements not observed. Atlas (the first vertebra) with rudimentary hypapophysis and not fused to axis, presacral vertebrae six in number, Vertebrae II–V bearing horizontally elongate hypapophyses, those on Vertebrae II and V oriented anteriorly; Vertebrae III–IV oriented horizontally; sacral diapophysis laterally dilated; coccyx not fused to sacrum; articulating with former by a double condyle and lacking lateral expansions, omosternum absent, pectoral girdle arciferal, with epicoracoids united to each other anteriorly and overlapping posteriorly (Fig. 2). Phalangeal formula of fingers 2-2-3-3; toes 2-2-3-4-3, terminal phalange obtusely curved, not truncate. Nasal bones of the skull large, about 1/3rd of frontoparietals and 1.25 times as large as orbital cavity. Maxillary and vomerine teeth absent.
Morphological variations
Adult females and males range between 25.2–27.4 mm and 21.8–25.5 mm, respectively. Measurements of paratypes are provided in Table 4. Dorsal colour in different shades of brown or reddish-brown. Intensity of inverted ‘V’-shaped pattern on dorsum variable. On one occasion, an abnormal specimen (WII-113) with a deformity was observed, with six digits, the first toe being preceded by a small additional toe on both feet. Fingers showed no such anomalies.
Description of calls
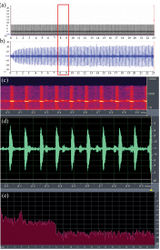
(Macaulay Library, Cornell Lab of Ornithology; voucher no: ML 174095). A calling male was observed on 24 November 2010 on the surface of leaves within bushes. Calls were composed of continuous syllables of “pip-pip-pip-pip-pip-” at a constant frequency of 8 kHz, without pause, lasting for 23 seconds, with mean amplitude of -3 db / 20 kU (Fig. 3). The call was composed of 198 pulses uttered within duration of 23 s, at a rate of 8 to 9 (mean = 8.6) pulses per second. Each pulse lasted for duration of 0.3 s (n = 198) with an interval of 8.5 s (n = 197) between two consecutive pulses.
Distribution
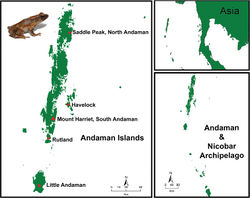
This species has been documented from five islands of the Andaman archipelago, namely, the South Andaman (Mt. Harriet), Rutland, Little Andaman, Havelock Island in the Ritchie’s Archipelago and North Andaman (Saddle Peak) (Fig. 4).
Vernacular name
‘Andaman bush toad’ is proposed as the common English name for this new species, indicating its arboreal habit and restricted distribution as understood currently.
Ecological notes
The new species is often seen on surface of leaves of herbaceous bushes. It is nocturnal and regularly seen year round. It was the third most common anuran in the islands (Harikrishnan and Vasudevan 2015[3]). The high abundance of this species seems to be the result of it occupying a narrow range of distribution and a unique niche of frogs belonging to the Old World tree frog family (Rhacophoridae), which are not known to occur on the Andaman Islands. All other anuran amphibians recorded from these islands are ground-dwelling, with the exception of Kaloula baleata ghoshi, which is semi-arboreal, and Ingerana charlesdarwini, which is known to use phytotelms for breeding and oviposition (Das 1998[4]). During day time, bush toads were found under leaf litter on the forest floor.
The Andaman bush toad emits a white, viscous, pungent smelling secretion from the parotoid glands when handled (Fig. 5a); the secretion seems to be toxic, as other frogs kept within the same bag as one of these toads suffered mortality. Breeding commences in June with the onset of the Southwest Monsoon. Males were observed to call from heights of ca. 1–1.5 m above ground while sitting on leaves of bushes. Amplexus is axillary (Fig. 5b), and females deposit ova in phytotelms, which are tree-holes at a height of about 1–1.5 m above the ground filled with rainwater. Tadpoles develop in these phytotelms. The shrub from which the tadpoles described here were collected, measured 19 cm diameter at breast height, and eggs were found in a depression of 6 cm depth, filled with water up to 3 cm. The tree hole was oval, measuring 5 × 3 cm across (Fig. 6a). The Andaman bush toad is widely distributed in islands where it occurs, and occupies forested habitats from 29–250 m asl, more common above 100 m asl and rarer at lower altitudes. The forest types in this elevation range include littoral, moist-deciduous, giant evergreen and montane stunted evergreen forests (Champion and Seth 1968[5]).
Conservation status
The Andaman bush toad is known from five islands: North Andaman (Saddle Peak National Park only), South Andaman, Rutland, Havelock (only in a small patch of wet forest towards the south of the island) and Little Andaman. Based on searches carried out using 21 bounded quadrats of 100 m2 each in these islands, the new species occurs at densities of 1.1 ± 0.37 toads per 100 m2 of forest floor (unpublished data). It is considered ‘Endangered’ based on IUCN Ver. 3.1. Second Edition (IUCN 2014[6]): criteria B.1 - extent of occurrence < 20000 km2 and B.1.a - severely fragmented population and known to exist at no more than 10 locations. A large array of invasive fauna in these Islands threatens the population of this toad. Additionally, stochastic events and anthropogenic pressures are potential threats to the species and its habitat.
Notes on larval development
(Fig. 6b–f) The clutch of ova in the phytotelm located in May 2011 at Rutland Island was monitored continuously until complete tadpole transformation. Unpigmented, early-stage larvae were observed on 2nd May 2011. A total of 73 hatchlings presumably from a single clutch could be counted in the phytotelm. Following subsequent rain showers four days later on 6th May, only 25 tadpoles of Stage 20 could be observed, the rest presumably washed out by overflow. At this stage, the tadpoles were translucent and colourless, but speckled with black, with white abdominal yolk region, dorsally positioned eyes and labia visible. On 19th May, i.e., 13 days later, two samples of Stages 30 and 35 were collected and preserved in formalin. Tadpoles of these stages had exposed hind limbs, lacking forelimb buds and were dull purplish-brown in colour, without a dorsal pattern. A week later, on 25th May, the tadpoles that developed into Stages of 41 and 43, were preserved. At these advanced stages, the tadpoles showed developed forelimbs, with expanded discs of fingers, more intense pigmentation on skin, and feeble barred pattern on limbs. The Stage 43 larva is briefly described: mouth positioned anteriorly, with prominent, keratinised pair of jaw sheaths; keratodont absent, eyes and nostrils positioned dorso-laterally (IO 1.46 mm), nostrils much closer to eyes than snout tip. Body depressed, head-body 1.5 times as long as broad (HBL: HBW 1.53), tail almost twice as long as head-body (tL/HBL 1.95) with well-developed caudal musculature. Measurements of the tadpoles are in Table 5.
| Stage | IOL | IND | NED | NSD | SS | SV | BL | TL | MBW | MTH | MTMW | TMH | ODD | VTL | DFH | VFH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 1 | 0.8 | 0.4 | 0.7 | 3.6 | 1.6 | 5 | 11.1 | 2.8 | 2.5 | 1.1 | 1.3 | 1.2 | 0.7 | 0.6 | 0.4 |
| 35 | 1.1 | 1.1 | 0.4 | 0.8 | 5 | 3.3 | 6.8 | 12.4 | 3.7 | 3.5 | 1.3 | 2 | 1 | 1.7 | 0.9 | 0.7 |
| 41 | 1 | 1 | 0.4 | 0.6 | 3.9 | 2.4 | 6.1 | 11.8 | 3.8 | 2.8 | 1.3 | 1.8 | 1.5 | 0.9 | 0.7 | 0.7 |
| 42 | 1.3 (±.20) | 1.1 | 0.4 (±.05) | 0.7 (±.10) | NA | NA | 7.3 (±.05) | 13.6 (±.05) | 3.5 (±.05) | 3.2 (±.10) | 1.8 (±.20) | 1.9 (±.15) | 1.3 (±.25) | NA | 0.9 (±.05) | 0.7 (±.05) |
| 43 | 1.5 (±.30) | 1.1 | 0.8 (±.20) | NA | NA | NA | 7.0 (±.10) | 9.4 (±1.05) | 3.8 (±.20) | 1.9 (±.35) | 1.2 (±.10) | 1.6 | 1.7 (±.10) | NA | 0.4 (±.05) | 0.3 |
Description of Tadpole
(Stage 35). Body tubular in dorsal and ovoid in lateral views, respectively (Fig. 6c). When viewed laterally, body dorsum is flattened and depressed medially; ventrally body slightly flattened at anterior end and convex towards posterior; body length 35% of total length; body attains maximum diameter in region immediately behind eyes. Snout broad and truncate in dorsal and pointed in lateral views, respectively. Eyes large; located and oriented dorso-laterally. Nostrils rounded with elevated rim, located almost midway but closer to eyes than snout, placed linear to eye in dorsal view; internarial distance subequal to interorbital distance. Spiracle sinistral and long with no inner wall; spiracle opening large; tube orientated postero-laterally, opening located approximately at midbody. Distance between spiracle and snout about 60% of body length. Intestinal coils not visible through the belly wall; vent tube medial. Tail tip broadly rounded; musculature linear till 1/3rd length of tail, after which it tapers. Dorsal fin slightly wider than ventral fin, originates posterior to body – tail junction and ventral fin at ventral terminus; both fins run parallel to tail muscle parallel through entire length of tail. Maximum tail height attained at about mid-length. Lateral line faintly visible. No glands observed on outer integument.
Oral disc positioned at terminal portion of body opening antero-ventrally (Fig. 6e); Rostral width of oral disc 27% body width, non-emarginate; entire oral disc visible dorsally; single row of seven to eight large marginal papillae present on lower labium and two to three on lateral corners; none present on upper labium; a single submarginal papilla located at each lateral corners; lower labium larger than upper labium. Denticle rows absent. Jaw sheaths well developed, heavily keratinised. Jaw sheaths completely serrated with minute serrations on lower jaw than upper jaw; suprarostrodont convex medially, longer than wide and lateral process of subequal height through length; infrarostrodont U-shaped.
Measurements (in mm; mean shown without parentheses and standard errors are shown in parentheses): Measurements of the seven tadpoles of various stage of development (Stages 30, 35, 41, 42 and 43) are presented in Table 5.
Colour
In life, dorsally, outer integument brown, with no melanopores. Ventrally, integument translucent but the gut was not visible; throat speckled. Both tail fins transparent with few melanophores. Laterally, tail muscle white with a few brown spots spread mainly at anterior region of tail. A completely transformed metamorph (SVL 10.6 mm; HL 4.23 mm) resembles adult in morphology, with an evident inverted ‘V’ mark on dorsum and transverse crossbars on limbs.
Morphological comparisons
Morphological and osteological characteristics of this new taxon are compared with members of other known Oriental bufonid genera below. The new taxon described here differs from the following known genera thus (only opposing character states in the genera being compared are mentioned):
Parapelophryne Fei, Ye & Jiang, 2003: type species– Nectophryne scalptus [current name combination: Parapelophryne scalpta (Liu and Hu 1973[7])]: Presence of eight presacral vertebrae and absence of parotoid glands (Fei et al. 2003[8]). The phylogenetic position of this taxon was assessed by Matsui et al. (2015)[9], who found it to be sister taxon to Bufo japonicus, thereby providing additional evidence for its distinctness from Blythophryne gen. n. described here. Distribution: Hainan, eastern China.
Pedostibes Günther, 1875: type species – Pedostibes tuberculosus Günther, 1875: Larger adult size (SVL 36.6–38.5 mm), presence of eight presacral vertebrae; short, rounded parotoid glands; tips of fingers dilated into truncated discs; small, numerous pigmented ova laid in strings, as in members of the genus Duttaphrynus and exotrophic larvae (Günther 1875, Inger et al. 1984[10], Fei et al. 2003[8], Matsui et al. 2007[11]). Currently, the genus Pedostibes is represented by five nominal species, which show a disjointed distribution pattern. The westernmost of all, Pedostibes tuberculosus, is the type species associated to the generic name (Günther 1875). Pedostibes kempi is known from the Garo Hills in Meghalaya, north-east India. Presently, Pedostibes kempi is considered congeneric, but differs in having a concealed tympanum. The remaining species, namely, Pedostibes rugosus, Pedostibes hosii and Pedostibes everetti occur in the Indo-Chinese and Indo-Malayan regions (Frost 2014[12]). Bocxlaer et al. (2009)[13], and more recently Ron et al. (2015)[14], in their phylogenetic studies, showed that the genus Pedostibes, as currently defined, does not constitute a monophyletic group. According to their study, the type species, Pedostibes tuberculosus does not show a close relationship with the south-east Asian Pedostibes hosii. On the other hand, they demonstrated that Pedostibes hosii is the sister taxon to Phrynoidis juxtasper. In addition, the generic placement of Pedostibes kempi is also uncertain owing to the inconsistencies in morphological characters associated with this taxon. Hence, resolving the higher level systematic status of the south-east Asian taxa currently allocated to the genus Pedostibes will require further study. Distribution: Western Ghats, Indochina, Malay Peninsula.
Bufoides Pillai & Yazdani, 1973: type species– Ansonia meghalayana [current name combination: Bufoides meghalayanus (Yazdani & Chanda, 1971); currently monotypic, but additional, unnamed species recognised; Das et al. 2009[15]]: Larger adult size (mean 42.9 mm, range 37–47 mm), absence of webbing and expanded discs in fingers, hidden tympanum, presence of cranial ridges and large, pigmented ova laid in strings, as in Duttaphrynus (Yazdni and Chanda 1972, Pillai and Yazdani 1973[16], Fei et al. 2003[8]), presence of seven presacral vertebrae, distinguish this taxon from the newly described genus. Distribution: Khasi Hills, Meghalaya, north-east India (Frost 2014[12]). Pelophryne Barbour, 1938: type species– Pelophryne albotaeniata Barbour, 1938: Presence of coccygeal expansions, absence of parotoid glands; fleshy manus with one phalange free of web and presence of seven (occasionally six) presacral vertebrae, urostyle fused to the sacrum and less number (n ≤ 30) of larger sized yolky eggs (Barbour 1938[17], Inger 1954[18], 1966[19]; Matsui et al. 2007[11]). Distribution: eastern Asia, Sundaland and the Philippines (Frost 2011).
Sabahphrynus Matsui, Yambun & Sudin, 2007: type species– Nectophryne maculata [current name combination: Sabahphrynus maculatus (Mocquard, 1890)]: Larger adult size (41.21 ± 2.5, 30.4–52.6), presence of eight presacral vertebrae, absence of tympanum and parotoid glands, absence of webbing between the fingers, over 50 eggs/ovary and absence of an external vocal sac in males (Matsui et al. 2007[11]). Distribution: endemic to Borneo (Frost 2014[12]).
Duttaphrynus Frost, Grant, Faivovich, Bain, Haas, Haddad, de Sá, Channing, Wilkinson, Donnellan, Raxworthy, Campbell, Blotto, Moler, Drewes, Nussbaum, Lynch, Green & Wheeler, 2006: type species– Bufo melanostictus [current name combination: Duttaphrynus melanostictus (Schneider, 1799)]: Large adult size (mean SVL 43.7 mm), presence of eight presacral vertebrae, presence of keratinised cephalic ridges in some species, presence of large, keratinised warts on the dorsum, absence of expanded discs in finger and toe tips, absence of webbing between the fingers, numerous black pigmented ova laid in long, continuous strings, exotrophic larvae and terrestrial habit (Dubois and Ohler 1999[20], Manamendra-Arachchi and Pethiyagoda 1998[21]). Particularly, the nomen Bufo camortensis (holotype – ZSI A 6955) erected for a species that is currently considered to represent Duttaphrynus melanostictus differs from the new taxon described here by its considerably large adult size [SVL – 67 mm (vs. much smaller mean adult size of 24 mm in Blythophryne gen. n.), presence of keratinised cephalic ridges and glandular tubercles on the body (vs. absent in Blythophryne gen. n.), absence of webbing between the fingers and dilated terminal discs in the digits (vs. present in Blythophryne gen. n.). Distribution: East Africa through the Middle East, India, Indochina, east to the Sundas till Bali (Frost 2014[12]).
Ansonia Stoliczka, 1870: type species – Ansonia penangensis Stoliczka, 1870: small to medium adult size (35–40 mm), absence of (or rudimentary) webbing between the fingers, presence of eight presacral vertebrae, absence of dilations in finger and toe tips, absence of parotoid glands, exotropic larvae with prominent oral discs and torrential stream dwelling habit (Inger 1960[22], Matsui et al. 2010[23]). Distribution: Indo-Malayan region and the Philippines (Frost 2014[12]).
Adenomus Cope, 1861: type species– Adenomus badioflavus Cope, 1861, a junior synonym of Bufo kelaartii [current name combination: Adenomus kelaarti (Günther, 1858)]: The genus Adenomus was resurrected from the synonymy of ‘Bufo’ by Manamendra-Arachchi and Pethiyagoda (1998)[21] to accommodate members of the ‘Bufo’ kelaarti group, characterised by smooth finger edges; differing from the new taxon described here by its larger adult size (mean SVL 38.4 mm), presence of seven presacral vertebrae, absence of sheath-like webbing between fingers, absence of expanded discs at digit tips, presence of cranial ridges and indistinct tympanum (in Adenomus kelaarti), terrestrial habit, pronounced sexual size dimorphism and unpigmented ova laid in long, continuous strings as in Duttaphrynus (Manamendra-Arachchi and Pethiyagoda 1998[21]; Haas 1999[24]; Meegaskumbura et al. 2015[25]). Distribution: endemic to Sri Lanka (Frost 2014[12]).
Ghatophryne Biju, Bocxlaer, Giri, Loader & Bossuyt, 2009: type species– Ansonia ornata [current name combination: Ghatophryne ornata (Günther, 1876)]: larger adult size (up to 35 mm SVL), characteristic reddish dorsal and ventral colouration, absence of parotoid glands, absence of webbing between the fingers, finger tips not dilated to discs and torrential stream dwelling habit (Biju et al. 2009[26]). Distribution: Central Western Ghats in the states of Kerala and Karnataka (Frost 2014[12]).
Xanthophryne Biju Bocxlaer, Giri, Loader & Bossuyt, 2009: type species– Bufo koynaensis [current name combination: Xanthophryne koynaensis (Soman, 1963)]: Larger adult size (up to 35.3 mm SVL), presence of characteristic chrome yellow patches along the flanks and sides of the abdomen, indistinct tympanum, weak, rounded parotoid glands, absence of webbing in fingers and discs in toes and fingers; large, pigmented ova laid in stagnant puddles on the ground (Biju et al. 2009[26]). Distribution: Known only from Northern Western Ghats in Maharashtra, India (Frost 2014[12]).
Leptophryne Fitzinger, 1843: type species – Bufo cruentatus [current name combination: Leptophryne cruentata (Tschudi, 1838)]: Dubois (1982)[27] resurrected the genus Leptophryne Fitzinger, 1843 as the senior synonym of Cacophryne Davis, 1935, which currently comprises two species – Leptophryne borbonica (Tschudi, 1838) and Leptophryne cruentata (Tschudi, 1838). Presence of eight presacral vertebrae; firmisternal pectoral girdle; elongate subarticular tubercles near the base of each toe, numerous pigmented eggs and exotrophic larvae (Fei et al. 2003[8]) distinguish it from Blythophryne beryet gen. et sp. n. Distribution: Sundaland (Frost 2014[12]).
Pseudobufo Tschudi, 1838: type species – Pseudobufo subasper Tschudi, 1838: Large body size, stout habitus; presence of seven presacral vertebrae (vs. six in Blythophryne gen. n.) completely (to the tip of Toe IV) webbed feet (vs. incomplete toe webbing in Blythophryne beryet gen. et sp. n.), fingers basally webbed; parotoid glands absent; dorsal, lateral and ventral skin surfaces with fine spinules, dorsoventrally depressed body with large, round warts and dorsally positioned nostrils (vs. lateral) distinguish it from the new genus described here (Fei et al. 2003[8]; Inger and Stuebing 2005[28]). Distribution: Sundaland.
Ingerophrynus Frost, Grant, Faivovich, Bain, Haas, Haddad, de Sá, Channing, Wilkinson, Donnellan, Raxworthy, Campbell, Blotto, Moler, Drewes, Nussbaum, Lynch, Green & Wheeler, 2006: type species– Bufo biporcatus [current name combination: Ingerophrynus biporcatus (Gravenhorst, 1829)]: Presence of seven presacral vertebrae (vs. six); absence of lateral dilations in the digit tips (vs. present); absence of webbing between the fingers (vs. present) and endotrophic (vs. exotrophic) larvae distinguish Blythophryne beryet gen. et sp. n. from Ingerophrynus. Distribution: Southern Yunnan, Indochina, the Malay Peninsula, the islands of Indo-Malaya, and Philippines.
Phrynoidis Fitzinger, 1843: type species – Bufo asper [current name combination: Phrynoidis asper (Gravenhorst, 1829)]: Large adult size (up to 100 mm SVL) presence of an omosternum, (vs. absent); presence of seven presacral vertebrae (vs. six); absence of lateral dilations of digit tips (vs. present) and exotrophic (vs. endotrophic) larvae distinguish this genus from the new genus Blythophryne gen. n. Distribution: Myanmar through western and peninsular Thailand, the Malay Peninsula, Sumatra, Borneo, and Java.
Apart from the above bufonid genera known from Oriental Asia, the new taxon described herein differs from the following central-west African genera:
Nectophryne Buchholz & Peters, 1875: type species – Nectophryne afra Buchholz & Peters, 1875 by the presence of eight presacral vertebrae (vs. six in Blythophryne beryet gen. et sp. n.); presence of lamelliform subdigital pads – a character unique to Nectophryne which is absent in the new taxon described here. Oriental forms including members of the genera Pedostibes and Pelophryne were attributed to Nectophryne earlier (Boulenger 1892[29], 1896[30], 1919[31]), until Barbour (1938)[17] redefined these genera.
Nectophrynoides Noble, 1926: type species – Nectophryne tornieri [current name combination: Nectophrynoides tornieri (Roux, 1906)]: The comparisons made here are restricted to the type species of Nectophrynoides because the genus is poorly defined and is composed of representatives with a broad spectrum of morphological and developmental characteristics. Though unique among bufonids in possessing an omosternum and a direct developmental mode (in Nectophrynoides viviparus), members of this genus are poorly diagnosed with respect to other genera (Menegon et al. 2004[32]). Larger adult size (SVL 21–30 mm), presence of expanded, truncate fingertips (vs. expanded and curved in Blythophryne beryet gen. et sp. n.), presence of eight presacral vertebrae (vs. 6 in Blythophryne beryet gen. et sp. n.) however, distinguish Nectophrynoides from the new taxon described here (see Tihen 1960[33]; Menegon et al. 2004[32]; Harper et al. 2010[34]).
Molecular phylogeny
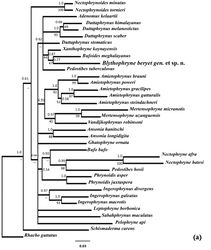
Multiple sequence alignment of the 16S homologous regions resulted in 498 conserved sites and 246 parsimoniously informative sites. In the phylogenetic analysis using both Maximum likelihood and Bayesian inference, the focal taxon showed a unique taxonomic position. The phylograms of both inference methods were similar (Fig. 7). Bufoides meghalayanus was found to be the closest taxon to the focal species, Blythophryne beryet gen. et sp. n. in the tree generated using 36 species from Asia and Africa but with relatively low support (Fig. 7a). However, when analysed with more of the Asian toads, it clearly separates out from species of Duttaphrynus, as well as, those of Xanthophryne and Bufoides (Fig. 7b, c). The average within-genus pairwise K2p distances at the partial 16S rRNA gene for all the described genera considered under this study was 0.0642, with 99% confidence interval (CI) of 0.0512–0.0687 (Table 6). The average pairwise k2p distance of the focal species with all other taxa at partial 16S rRNA gene considered here was 0.103, with a 99% CI of 0.096–0.113, strongly supporting its distinctiveness and unique phylogenetic position within the Bufonids. Similarly, for partial 12S rRNA gene, the average within-genus pairwise K2p distances for all described genera was 0.0495, with the 99% CI of 0.0387–0.0603. The average pairwise k2p distance of the focal species with all other taxa at partial 12S rRNA gene was 0.0783, with a 99% CI of 0.072–0.085. Both tree-based and distance-based analyses clearly indicate the uniqueness of its phylogenetic position. Thus, the rDNA typing strongly suggest the new taxon as a candidate to be named as a new genus/species.
| 16S/12S k2p uncorrected pair-wise distance estimates | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa (Genus*/Species) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
| 1 | Amietophrynus | 0.068/0.044 | 0.060 | 0.049 | 0.072 | 0.067 | 0.094 | 0.066 | 0.059 | 0.074 | 0.071 | 0.066 | 0.049 | 0.077 | 0.043 | 0.100 | 0.103 | 0.067 | 0.063 | 0.043 | 0.037 | 0.071 |
| 2 | Ansonia | 0.106 | 0.066/0.060 | 0.061 | 0.086 | 0.083 | 0.115 | 0.079 | 0.066 | 0.081 | 0.073 | 0.065 | 0.071 | 0.104 | 0.061 | 0.114 | 0.097 | 0.067 | 0.067 | 0.055 | 0.063 | 0.085 |
| 3 | Duttaphrynus | 0.092 | 0.089 | 0.049/ 0.038 | 0.070 | 0.063 | 0.099 | 0.067 | 0.060 | 0.065 | 0.076 | 0.065 | 0.054 | 0.084 | 0.045 | 0.098 | 0.099 | 0.074 | 0.054 | 0.045 | 0.049 | 0.077 |
| 4 | Ingerophrynus | 0.090 | 0.084 | 0.074 | 0.056/0.077 | 0.077 | 0.102 | 0.075 | 0.082 | 0.073 | 0.085 | 0.071 | 0.081 | 0.093 | 0.065 | 0.113 | 0.111 | 0.078 | 0.071 | 0.062 | 0.075 | 0.085 |
| 5 | Mertensophryne | 0.095 | 0.100 | 0.099 | 0.090 | 0.059/ 0.061 | 0.098 | 0.079 | 0.066 | 0.075 | 0.083 | 0.075 | 0.079 | 0.094 | 0.067 | 0.108 | 0.114 | 0.083 | 0.079 | 0.059 | 0.065 | 0.073 |
| 6 | Nectophryne | 0.143 | 0.141 | 0.127 | 0.139 | 0.140 | 0.089/0.049 | 0.092 | 0.095 | 0.098 | 0.107 | 0.093 | 0.098 | 0.108 | 0.073 | 0.146 | 0.136 | 0.102 | 0.083 | 0.090 | 0.102 | 0.081 |
| 7 | Nectophrynoides | 0.097 | 0.091 | 0.077 | 0.082 | 0.099 | 0.132 | 0.021/0.015 | 0.068 | 0.084 | 0.069 | 0.069 | 0.069 | 0.085 | 0.051 | 0.119 | 0.089 | 0.061 | 0.077 | 0.034 | 0.077 | 0.069 |
| 8 | Pedostibes | 0.091 | 0.086 | 0.066 | 0.083 | 0.104 | 0.115 | 0.074 | 0.076/0.065 | 0.075 | 0.071 | 0.067 | 0.063 | 0.085 | 0.051 | 0.130 | 0.107 | 0.068 | 0.069 | 0.048 | 0.063 | 0.071 |
| 9 | Phrynoidis | 0.086 | 0.084 | 0.073 | 0.082 | 0.102 | 0.135 | 0.080 | 0.080 | 0.039/0.085 | 0.089 | 0.079 | 0.091 | 0.108 | 0.063 | 0.123 | 0.119 | 0.085 | 0.069 | 0.071 | 0.085 | 0.081 |
| 10 | Ghatophryne ornata | 0.092 | 0.104 | 0.075 | 0.086 | 0.103 | 0.148 | 0.088 | 0.083 | 0.087 | n/a | 0.061 | 0.081 | 0.102 | 0.053 | 0.124 | 0.097 | 0.081 | 0.077 | 0.057 | 0.065 | 0.086 |
| 11 | Leptophryne borbonica | 0.103 | 0.107 | 0.095 | 0.096 | 0.117 | 0.121 | 0.099 | 0.082 | 0.099 | 0.092 | n/a | 0.073 | 0.085 | 0.045 | 0.106 | 0.085 | 0.069 | 0.053 | 0.061 | 0.061 | 0.069 |
| 12 | Vandijkophrynus robinsoni | 0.083 | 0.102 | 0.063 | 0.071 | 0.082 | 0.132 | 0.077 | 0.077 | 0.073 | 0.064 | 0.102 | n/a | 0.072 | 0.053 | 0.110 | 0.097 | 0.073 | 0.072 | 0.037 | 0.049 | 0.085 |
| 13 | Schismaderma carens | 0.096 | 0.099 | 0.076 | 0.088 | 0.086 | 0.132 | 0.082 | 0.079 | 0.095 | 0.097 | 0.107 | 0.092 | n/a | 0.089 | 0.110 | 0.110 | 0.089 | 0.069 | 0.077 | 0.081 | 0.094 |
| 14 | Bufo bufo | 0.102 | 0.113 | 0.078 | 0.107 | 0.116 | 0.134 | 0.085 | 0.088 | 0.082 | 0.087 | 0.105 | 0.076 | 0.105 | n/a | 0.110 | 0.085 | 0.057 | 0.057 | 0.034 | 0.045 | 0.057 |
| 15 | Sabahphrynus maculatus | 0.092 | 0.085 | 0.077 | 0.071 | 0.097 | 0.124 | 0.093 | 0.079 | 0.081 | 0.082 | 0.093 | 0.087 | 0.082 | 0.104 | n/a | 0.145 | 0.114 | 0.110 | 0.102 | 0.106 | 0.123 |
| 16 | Pelophryne api | 0.108 | 0.105 | 0.102 | 0.092 | 0.100 | 0.155 | 0.106 | 0.097 | 0.106 | 0.100 | 0.113 | 0.100 | 0.122 | 0.126 | 0.112 | n/a | 0.089 | 0.093 | 0.068 | 0.101 | 0.093 |
| 17 | Bufoides meghalayanus | 0.088 | 0.091 | 0.055 | 0.080 | 0.109 | 0.133 | 0.073 | 0.064 | 0.069 | 0.078 | 0.095 | 0.062 | 0.087 | 0.071 | 0.087 | 0.105 | n/a | 0.073 | 0.041 | 0.069 | 0.069 |
| 18 | Adenomus kelaartii | 0.091 | 0.091 | 0.065 | 0.084 | 0.107 | 0.136 | 0.070 | 0.071 | 0.070 | 0.066 | 0.098 | 0.059 | 0.090 | 0.083 | 0.085 | 0.103 | 0.062 | n/a | 0.061 | 0.069 | 0.065 |
| 19 | Xanthophryne koynayensis | 0.084 | 0.078 | 0.054 | 0.070 | 0.098 | 0.129 | 0.072 | 0.056 | 0.077 | 0.073 | 0.087 | 0.059 | 0.082 | 0.078 | 0.078 | 0.083 | 0.039 | 0.057 | n/a | 0.049 | 0.049 |
| 20 | Rhaebo guttatus | 0.111 | 0.085 | 0.092 | 0.088 | 0.104 | 0.136 | 0.089 | 0.083 | 0.086 | 0.087 | 0.105 | 0.092 | 0.088 | 0.114 | 0.094 | 0.112 | 0.092 | 0.085 | 0.075 | n/a | 0.086 |
| 21 | Blythophryne beryet gen. et sp. n. | 0.103 | 0.119 | 0.089 | 0.098 | 0.118 | 0.165 | 0.098 | 0.102 | 0.088 | 0.099 | 0.112 | 0.092 | 0.101 | 0.105 | 0.106 | 0.112 | 0.080 | 0.082 | 0.075 | 0.109 | n/a |
Original Description
- Chandramouli, S; Vasudevan, K; Harikrishnan, S; Dutta, S; Janani, S; Sharma, R; Das, I; Aggarwal, R; 2016: A new genus and species of arboreal toad with phytotelmonous larvae, from the Andaman Islands, India (Lissamphibia, Anura, Bufonidae) ZooKeys, (555): 57-90. doi
Images
|
Other References
- ↑ Das I (1999) Biogeography of the amphibians and reptiles of the Andaman and Nicobar Islands. In: Ota H (Ed.) Tropical island herpetofauna. Origin, current diversity and conservation. Elsevier Science B.V. Amsterdam, 43–77.
- ↑ Das I (2004) Herpetology of an antique land: the history of herpetological explorations and knowledge in India and south Asia. Bonner Zoologische Beiträge 52: 215–229.
- ↑ Harikrishnan S, Vasudevan K (2015) The devil is in the detail: estimating species richness, density, and relative abundance of tropical island herpetofauna. BMC Ecology 15: 18. doi: 10.1186/s12898-015-0049-5
- ↑ Das I (1998) A remarkable new species of ranid (Anura: Ranidae) with phytotelmonous larvae, from Mount Harriet, Andaman Islands. Hamadryad 23: 41–49.
- ↑ Champion H, Seth S (1968) A revised survey of the forest types of India. Forest Research Institute, Dehradun, 404 pp.
- ↑ IUCN (2014) IUCN Red List of Threatened Species. Version 2013.2. http://www.iucnredlist.org [accessed 2 June 2014]
- ↑ Liu C, Hu S (1973) On collections of amphibians from Hainan Island. Acta Zoologica Sinica/ Dong Wu Xue Bao 19: 385–404.
- ↑ 8.0 8.1 8.2 8.3 8.4 Fei L, Ye C, Jiang J (2003) A new bufonid genus Parapelophryne from China (Amphibia, Anura). Acta Zootaxonomica Sinica 28: 762–766.
- ↑ Matsui M, Eto K, Lau M, Liu W, Nishikawa K (2015) Unexpected phylogenetic position of Parapelophryne among Southeast Asian bufonids as revealed by mitochondrial DNA sequence (Amphibia, Anura, Bufonidae). Current Herpetology 34: 182–187. doi: 10.5358/hsj.34.182
- ↑ Inger R, Shaffer H, Koshy M, Bakde R (1984) A report on a collection of amphibians and reptiles from the Ponmudi, Kerala, South India. Journal of the Bombay Natural History Society 81: 406–427.
- ↑ 11.0 11.1 11.2 Matsui M, Yambun P, Sudin A (2007) Taxonomic relationships of Ansonia anotis Inger, Tan, Yambun, 2001 and Pedostibes maculatus (Mocquard, 1890), with a description of a new genus (Amphibia, Bufonidae). Zoological Science 24: 1159–1166. doi: 10.2108/zsj.24.1159
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 Frost D (2014) Amphibian Species of the World: an Online Reference. Version 6. http://research.amnh.org/vz/herpetology/amphibia [accessed 2014-31-05]
- ↑ Bocxlaer I, Biju S, Loader S, Bossuyt F (2009) Toad radiation reveals into-India dispersal as a source of endemism in the Western Ghats-Sri Lanka biodiversity hotspot. BMC Evolutionary Biology 9: 131. doi: 10.1186/1471-2148-9-131
- ↑ Ron S, Mueses-Cisneros J, Gutiérrez-Cárdenas P, Rojas-Rivera A, Lynch R, Rocha C, Galarza G (2015) Systematics of the endangered toad genus Andinophryne (Anura: Bufonidae): phylogenetic position and synonymy under the genus Rhaebo. Zootaxa 3: 347–366. doi: 10.11646/zootaxa.3947.3.3
- ↑ Das I, Rangad D, Tron R, Deuti K, Hooroo R (2009) Rediscovery of the endangered Khasi Hills rock toad, Bufoides meghalayana in Meghalaya, Northeastern India. Froglog 92: 1–4.
- ↑ Pillai R, Yazdani G (1973) Bufoides, a new genus for the rock-toad, Ansonia meghalayana Yazdani & Chanda, with notes on its ecology and breeding habits. Journal of the Zoological Society of India 25: 65–70.
- ↑ 17.0 17.1 Barbour T (1938) Notes on the “Nectophryne”. Proceedings of the Biological Society of Washington 51: 191–196.
- ↑ Inger R (1954) Systematics and zoogeography of Philippine Amphibia. Fieldiana Zoology 33: 183–531. doi: 10.5962/bhl.title.5571
- ↑ Inger R (1966) The systematics and zoogeography of the Amphibia of Borneo. Fieldiana Zoology 52: 1–402.
- ↑ Dubois A, Ohler A (1999) Asian and Oriental toads of the Bufo melanostictus, Bufo scaber and Bufo stejnegeri groups (Amphibia, Anura): a list of available names and redescription of some name-bearing types. Journal of South Asian Natural History 4: 133–180.
- ↑ 21.0 21.1 21.2 Manamendra-Arachchi K, Pethiyagoda R (1998) A synopsis of the Sri Lankan Bufonidae (Amphibia: Anura) with description of two new species. Journal of South Asian Natural History 3: 213–246.
- ↑ Inger R (1960) A review of the Oriental toads of the genus Ansonia Stoliczka. Fieldiana Zoology 39: 473–503. doi: 10.5962/bhl.title.2697
- ↑ Matsui M, Tominaga A, Liu W, Khonsue W, Grismer L, Diesmos A, Das I, Sudin A, Yambun P, Yong H, Sukumaran J, Brown R (2010) Phylogenetic relationships of Ansonia from Southeast Asia inferred from mitochondrial DNA sequences: systematic and biogeographic implications (Anura: Bufonidae). Molecular Phylogenetics and Evolution 54: 561–570. doi: 10.1016/j.ympev.2009.08.003
- ↑ Haas W (1999) Zur Biologie von Bufo kelaartii Günther, 1859. Elaphe 7: 16–19.
- ↑ Meegaskumbura M, Senevirathne G, Wijayathilaka N, Jayawardena B, Bandara C, Manamendra-Arachchi K, Pethiyagoda R (2015) The Sri Lankan torrent toads (Bufonidae: Adenominae: Adenomus): species boundaries assessed using multiple criteria. Zootaxa 3911(2): 245–261. doi: 10.11646/zootaxa.3911.2.6
- ↑ 26.0 26.1 Biju S, Bocxlaer I, Giri V, Loader S, Bossuyt F (2009) Two new endemic genera and one new species of toad (Anura: Bufonidae) from the Western Ghats of India. BMC Research Notes 2: 241. doi: 10.1186/1756-0500-2-241
- ↑ Dubois A (1982) Leptophryne Fitzinger, 1843, a senior synonym of Cacophryne Davis, 1935 (Bufonidae). Journal of Herpetology 16: 173–174. doi: 10.2307/1563811
- ↑ Inger R, Stuebing R (2005) A field guide to the frogs of Borneo. Second edition. Natural History Publications (Borneo) Kota Kinabalu, 1–201.
- ↑ Boulenger G (1892) An account of the reptiles and batrachians collected by Mr. C. Hose on Mt. Dulit, Borneo. Proceedings of the Zoological Society of London 1892: 505–508.
- ↑ Boulenger G (1896) Descriptions of two new batrachians obtained by Mr. Everett on Mt. Kina Balu, North Borneo. Annals and Magazine of Natural History (Series 6) 17: 449–450.
- ↑ Boulenger G (1919) Descriptions of three new batrachians from the Garo Hills, Assam. Records of the Indian Museum 16: 207–208.
- ↑ 32.0 32.1 Menegon M, Salvidio S, Loader S (2004) Five new species of Nectophrynoides Noble, 1926 (Amphibia: Anura, Bufonidae) from the Eastern Arc Mountains, Tanzania. Tropical Zoology 17: 97–121. doi: 10.1080/03946975.2004.10531201
- ↑ Tihen J (1960) Two new genera of African bufonids, with remarks on the phylogeny of related genera. Copeia 1960: 225–233. doi: 10.2307/1439662
- ↑ Harper E, Measey G, Patrick D, Menegon M, Vonesh J, Swilla I (2010) Field guide to amphibians of the Eastern Arc Mountains and coastal forests of Tanzania and Kenya. Camerapix Publishers International, Nairobi, 320 pp.