Andesobia jelskii
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Schmidt2011ZooKeys149, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Schmidt2011ZooKeys149">{{Citation See also the citation download page at the journal. |
Ordo: Lepidoptera
Familia: Erebidae
Genus: Andesobia
Name
Andesobia jelskii (Oberthür, 1881) comb. n. – Wikispecies link – Pensoft Profile
- Arctia jelskii Oberthür 1881[1]: 33, pl. X, f. 3. Male holotype [BMNH]. Type locality: [Peru], Junin.
- Mallocephala imitatrix Rothschild 1922[2]: 493, comb. n, syn. n. 16 male and 5 female syntypes [BMNH]. Type locality: Peru, Junin.
- Mallocephala imitatrix ab. griseola Rothschild 1922[2]: 493, comb. n, syn. n. Holotype male [BMNH]. Type locality: Peru, Junin.
- Mallocephala imitatrix ab. luteola Rothschild 1922[2]: 493, comb. n, syn. n. 7 female syntypes [BMNH]. Type locality: Peru, Junin.
Material examined
We examined over 150 specimens, obtained through four successive lab-reared generations originating from Peru, Junin region, Huicuash E of Tarma, 11°23'S, 75°53'W, 4100 m. Vouchers are deposited in CDFM, ZSM, CNC, CPG, CTN. Two specimens were included for DNA barcode analysis, voucher numbers CNC LEP 68032 (no GenBank accession number available) and CNC LEP 68033 (GenBank accession # HM416594) [CNC].
Diagnosis and re-description
Andesobia jelskii was omitted from the catalogue of Neotropical Arctiinae (Watson and Goodger 1982), probably because Hampson (1901)[3] placed it in the Palaearctic genus Ocnogyna. This species is most similar to Andesobia flavata and Andesobia boliviana, but lacks any trace of transverse forewing lines and has an uninterrupted ochre costal band, which is interrupted by the antemedial and postmedial lines in other Andesobia species; the hindwing marginal band has a diffuse inner border and is much wider than in other species of Andesobia, extending at least to the discal area. Internally, the male valve is shorter and wider, appropximately 6 × longer than the narrowest diameter compared to 6.7–8.0 × longer in Andesobia flavata.
A detailed morphological description is given in the generic account of Andesobia, and the following description addresses characters specific to Andesobia jelskii. Male. Head – antenna (Fig. 9a) with posterior rami 1.6–1.9 × segment length, longest anterior rami 1.4–1.8 × segment length; eye elliptical, 1.4–1.6 × as high as wide. Thorax –vestiture and legs black brown, dorsum of femur ochre. Forewing – forewing length average 11 mm, range 8–12 mm; ground colour brownish grey with yellowish-ochre costal band varying to entirely dark brownish grey or plae ochre grey (type of luteola), indistinct black discal spot, other markings obsolete ventrally with paler yellowish ochre ground colour. Hindwing – ground colour yellowish ochre with broad, diffusely bordered grey-brown marginal band over distal third of wing, varying to entirely dark brownish grey; brownish, crescentic discal spot small but well defined; ventrally with dark markings less saturated. Abdomen – segments A1–A3 brownish black, remaining segments ochre subdorsally, with brownish-black dorsal line; ventrally entirely ochre. Genitalia (Figs 9b,c) – valve digitate, slightly flattened laterally and narrowing slightly medially; equal in length to uncus-tegumen complex; vinculum semicircular, saccus v-shaped, similar in length to uncus; aedeagus relatively large and stout, 3 × longer than wide, 1.5 × as long as width of genital capsule, curving dorsad 25–30°, proximal end approximately ⅓ narrower than apex; coecum 1/6–1/8 length of aedeagus, directed slightly ventrad; vesica directed dorso-distad, globose, finely spiculate, with poorly differentiated apical diverticulum. Female (Figs 5, 10, 13, 16). Described above in the genus account for Andesobia; differing externally from Andesobia sanguinea by the lack of yellowish-orange flush present in Andesobia sanguinea, particuarly on the ventral and lateral surfaces of the abdomen.
Immature stages
Egg – almost spherical, poles only very weakly flattened; micropyle very weakly sculptured, barely visible; ivory white changing to dark greyish white prior to hatching. Larva – 1st instar larva initially translucent white, becoming opaque white; setae black, yellow orange prior to molting. 2nd instar integument black, more densely setose than 1st instar. 3rd instar with jet black setae, except rusty brown on A2–A5; spiracles white. 4th instar (Figs 15a, b) with verrucae more pronounced than in previous instar; setae jet black with silver sheen apically, somewhat lighter smoky grey subventrally; colour of setae polymorphic in last instar, either with A2–A5 yellowish orange and segments A6–A8 with subdorsal and lateral silvery-white setae mixed in (Fig. 15a), or with orange setae very dark brown to black (Fig. 15b); when mature, female larva about twice as large as male larva. Pupa – cremaster short, penicillate, head compact with short, stiff setae (Fig. 12). Cocoon spherical to ovoid, reddish brown to dull brown, consisting of a single, thin and flimsy layer with incorporated larval setae (Fig. 16).
Biology and distribution
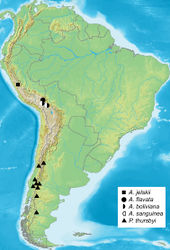
Eggs whitish, turning dark grey three days prior to hatching, hatching in about 10 days. First instar larvae initially feed on the tissue of the dead or dying female, then leave the cocoon in search of plant material. Duration of the first instar is six days, second instar five to six days. Taraxacum F.H. Wigg. and lawn grass (Poa L.) are both acceptible food plants in captivity, suggesting that larvae are polyphagous in nature. Notably, larvae emit an unpleasant odour of decay when disturbed. In late instars, female larvae are twice as large as male larvae. During the first three instars, larvae avoided sunlight, but the last two instars showed increased tolerance to sunlight, possibly to accelerate development. Cannibalism was not observed even at higher densities. Males pupated sooner than females, but the pupal stage is shorter in females lasting only a few days, so emergence of both sexes is more or less synchronous. Cocoons (Fig. 16) were spun between leaves of the food plants near the ground. The moths emerge in the morning, with relatively fast expansion of the wings. Females remain in the loosely-spun cocoon, and presumably emit mating pheromones from within the thin cocoon soon after emerging from the pupa, because males dig through the loose silk webbing to enter and mate inside the cocoon (Fig. 16). The pair remains in copula for several hours, after which the male leaves the cocoon, and the female deposits about 50 eggs inside the cocoon. Males are diurnal and fly rapidly during sunny periods. Reared cohorts of Andesobia jelskii displayed an unequal sex ratio of about 5 female: 3 male; female microptery and a female-biased sex ratio is associated with parthenogenesis in other families (Heterogynidae, Psychidae, Lymantriinae: Teia Hübner), and Andesobia may also be capable of parthenogenesis, which has not been documented in the Arctiinae. Andesobia jelskii is currently known only from the Junin region of Peru (Fig. 17), at 4100 m elevation in the Puna grassland ecoregion of the central Andes (Fig. 18). Like other members of the genus, the flight period is early in the year (January), in the middle of the four-month wet season.
Taxon Treatment
- Schmidt, B; Freina, J; 2011: Generic placement of the Neotropical species of “ Phragmatobia” (Erebidae, Arctiinae), with a remarkable matrivorous species from the Peruvian Andes ZooKeys, 149: 69-88. doi
Other References
- ↑ Oberthür C (1881) Etudes d’Entomologie (VI). 115 pp + 20 plates.
- ↑ 2.0 2.1 2.2 Rothschild W (1922) A preliminary list of the Arctiinae of Para, Brazil, and a few from other localities. Ann. Mag. nat. Hist. 9 (53): 457-494. doi: 10.1080/00222932208632699
- ↑ Hampson G (1901) Catalogue of the Arctiadae (Arctianae) and Agaristidae in the collection of the British Museum (Nat. Hist.). – Catalogus Lepidopteraorum Phalaenae of the British Museum, 3: 1–690, plates 36–54.
Images
|