Omphale clypealis
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Hansson2012ZooKeys232, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Hansson2012ZooKeys232">{{Citation See also the citation download page at the journal. |
Ordo: Hymenoptera
Familia: Eulophidae
Genus: Omphale
Name
Omphale clypealis Hansson & Shevtsova, 2012 (Thomson) – Wikispecies link – Pensoft Profile
- Derostenus (Secodes) clypealis Thomson, 1878:270. Lectotype female in LUZM, examined.
- Secodes clypealis (Thomson), Dalla Torre (1898)[1].
- Omphale clypealis (Thomson), Graham (1963)[2].
Material
Type material. Lectotype female, type no. 116:1 in LUZM. Additional material. 153♀ 3♂: Denmark 2♀ (LUZM, ZMUC), France 2♀ (RMNH), Germany 1♀ 1♂ (BMNH, RMNH), Hungary 26♀ (BMNH, CH), Netherlands 1♀ (RMNH), Russia 1♀ (BMNH), Spain 2♀ 2♂ (RMNH), Sweden 111♀ (BMNH, CH, LUZM, NHRS, RMNH), United Kingdom 7♀ (BMNH).
Diagnosis
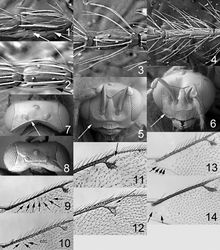
Clypeus yellowish white (Figs 324, 325); female antennal clava 3-segmented (Fig. 329); forewing speculum open below (Fig. 326), admarginal setae 10–16 arising mainly from membrane, radial cell bare; femora and tibiae metallic, tarsi dark brown (Fig. 321).
Description
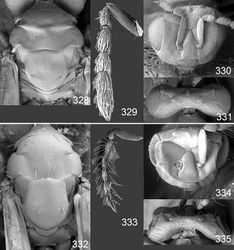
Female. Length of body 1.3–1.7 mm. Antenna dark brown to black with metallic tinges; pedicel + flagellum 1.5× as long as distance between eyes; first flagellomere 1.2× as long and 1.0× as wide as second flagellomere (Fig. 329); flagellomeres 2–4 ventrally with a single set of setae attach close to base and reaching beyond apex of flagellomere attached to; longitudinal sensilla on flagellomeres distinctly shorter than flagellomere attached to; clava 3-segmented. Face golden green with blue metallic tinges (Fig. 324), with strong reticulation (Fig. 330); clypeus yellowish white, smooth, trapezoid, 2.0× as wide as high; gena bluish green metallic; lower frons bluish green metallic, with raised and strong reticulation; interscrobal area reticulate; antennal scrobes join on frontal suture; frontal suture V-shaped; upper frons bluish green metallic, with raised and weak reticulation; vertex golden green with blue metallic tinges, with raised and weak reticulation (Fig. 331). Occipital margin rounded (Fig. 331).
Mesoscutum bluish green metallic with golden tinges (Fig. 322), with raised reticulation (Fig. 328), midlobe with two pairs of setae; notauli as indistinct depressions in posterior ½. Scutellum golden green with blue metallic tinges (Fig. 322), with raised reticulation (Fig. 328), 1.1× as long as wide, with anterior margin smoothly curved forwards. Axillae golden (Fig. 322). Dorsellum golden green (Fig. 322), with weak reticulation (Fig. 328), 0.3× as long as wide, and 0.4× as long as length of median propodeum. Entire lateral mesosoma bluish green metallic (Fig. 321), with or without golden tinges; transepimeral sulcus curved forwards. Propodeum bluish green metallic (Fig. 322), with very weak reticulation, to smooth (Fig. 328); propodeal callus with two setae. Coxae, femora and tibiae bluish green metallic with yellowish white “knees” (Fig. 321); tarsi dark brown; midleg with first tarsomere 0.2× as long as length of tarsus. Forewing transparent, veins and setae dark brown (Fig. 326); speculum open; admarginal setae 10–16, arising mainly from wing membrane; radial cell bare; postmarginal vein 0.9× as long as stigmal vein; stigmal vein elongate. Hind wing transparent, apex rounded (Fig. 326). Forewing WIP (Fig. 327) with apical ½ yellow and basal ½ blue and with a narrow band in magenta between these areas, also with a narrow blue line from stigmal vein towards apical margin of wing.
Petiole black. Gaster bluish green metallic with posterior ½ of tergite 1 and tergites 2+3 golden purplish, smooth, elongate and 1.3–1.4× as long as length of mesosoma; 7th tergite 0.1× as long as length of gaster.
Male. Length of body 1.1–1.4 mm. Features as in female except as follows. Antenna with scape with basal ½ yellowish white and apical ½ dark brown; pedicel + flagellum 2.5× as long as distance between eyes; flagellomeres with scattered setae (Fig. 333); clava 1-segmented. Face golden green (Fig. 325), clypeus 2.2× as wide as high; gena golden green; lower frons golden green; antennal scrobes join frontal suture separately (Fig. 334); upper frons golden green, with weak reticulation; vertex golden green.
Mesoscutum golden green with blue metallic tinges (Fig. 323). Scutellum golden green (Fig. 323). Axillae golden green (Fig. 323). Dorsellum bluish green metallic (Fig. 323), 0.6× as long as length of median propodeum. Propodeum golden green (Fig. 323). Forewing hyaline, veins pale brown; admarginal setae 12; postmarginal vein 0.6× as long as stigmal vein.
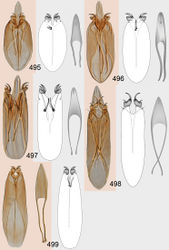
Petiole black. Gaster golden green with blue metallic tinges, smooth. Phallobase and aedeagus as in Fig. 495.
Host
Dasineura brassicae (Diptera: Cecidomyiidae) (e.g. Gijswijt 1976[3]). See above in the introduction for more information on the biology of this species.
Distribution
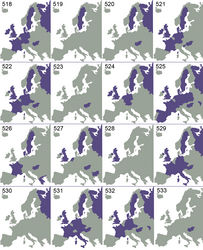
Czech Republic (Bouček and Askew 1968[4]), Denmark (Bakkendorf 1955[5]), France (new record), Germany (Buhl 1960[6]), Hungary (Erdös 1956[7]), Moldova (Bouček 1965[8]), Netherlands (Gijswijt 1976[3]), Poland (Miczulski 1968[9]), Russia (new record), Spain (new record), Sweden (Thomson 1878[10]), Switzerland (Büchi and Keller 1994[11]), United Kingdom (Graham 1963[2]) (Fig. 525).
Taxon Treatment
- Hansson, C; Shevtsova, E; 2012: Revision of the European species of Omphale Haliday (Hymenoptera, Chalcidoidea, Eulophidae) ZooKeys, 232: 1-157. doi
Other References
- ↑ Dalla Torre K (1898) Catalogus Hymenopterorum hucusque descriptorum systematicus et synonymicus, V Chalcididae et Proctotrupidae. Leipzig. 598 pp.
- ↑ 2.0 2.1 Graham M (1963) Additions and corrections to the British list of Eulophidae (Hym., Chalcidoidea), with descriptions of some new species. Transactions of the Society for British Entomology 15: 167-275.
- ↑ 3.0 3.1 Gijswijt M (1976) Notes on biology and distribution of the genus Omphale Haliday, 1833, with descriptions of two new species (Insecta, Hymenoptera, Eulophidae). Bulletin, Zoologisch Museum, Universiteit van Amsterdam 5: 77-84.
- ↑ Bouček Z, Askew R (1968) Palearctic Eulophidae, excl. Tetrastichinae. Index of Entomophagous insects. Le Francois, Paris, 254 pp.
- ↑ Bakkendorf O (1955) Notes on Icelandic and Greenlandic Chalcidoideous Hymenoptera. Entomologiske Meddelelser 27: 135-162.
- ↑ Buhl C (1960) Beobachtungen über vermehrtes Schadauftreten der Kohlschotenmücke (Dasyneura brassicae Winn.) an Raps und Rübsen in Schleswig-Holstein. Nachrichtenblatt Deutschen Pflanzschutzdienst 12: 1-6.
- ↑ Erdös J (1956) Additamente ad cognitionem faunae Chalcidoidarum in Hungaria et regionibus finitimis VI 19 Eulophidae. Folia Entomologica Hungarica (s.n. ) 9: 1-64.
- ↑ Bouček Z (1965) A review of the Chalcidoid fauna of the Moldavian S.S.R., with descriptions of new species (Hymenoptera). Acta Faunistica Entomologica Musei National Pragae 11: 5-38.
- ↑ Miczulski B (1968) Community studies on Hymenoptera found on Brassica napus L. Part VI. Chalcidoidea. Polskie Pismo Entomologiczne 38: 341-385.
- ↑ Thomson C (1878) Hymenoptera Scandinaviae. Lundae 5: 1-307. doi: 10.1038/005001a0
- ↑ Büchi R, Keller S (1994) Die Parasitierung der Kohlschotengallmücke durch Nützlinge. Agrarforschung 1: 400-402.
Images
|