Microporella verrucosa
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Martino2021ZooKeys1053, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Martino2021ZooKeys1053">{{Citation See also the citation download page at the journal. |
Ordo: Cheilostomatida
Familia: Microporellidae
Genus: Microporella
Name
Microporella verrucosa (Peach, 1868) – Wikispecies link – Pensoft Profile
- Eschara verrucosa Peach, 1868: 116.
- Diporula verrucosa (Peach): Hincks, 1880: 220, pl. 31, figs 1, 2; Gautier, 1962: 176; Zabala, 1986: 501, fig. 174, pl. 15A, B; Hayward and Ryland, 1979: 226, fig. 97; Hayward and Ryland, 1999: 302, figs 138C, D, 139; André et al. 2014[1]: 225, 5 figs; Rosso et al. 2014[2]: table 2, fig. 3A–C.
- Microporella (Diporula) verrucosa (Peach): Neviani, 1896a: 105; 1896b: 24.
Examined material
Italy • 2 colonies and 10 fragments (living), 17 colonies and 62 fragments (dead), some very large, some regenerated and twisted; Ionian Sea, SE Sicily, Ciclopi Islands MPA; Ciclopi 2000 cruise; samples 2G, 3H, 4E, 6H, 8F, 8H, 8I, 9G, 10G, 12E, 12F, 12G, 13H, 14G; 37°32'39"–37°34'31"N, 15°8'58"–15°11'1"E; 63–92.5 m; Jul. 2000; A. Rosso leg.; dredging; C, DC, DE, and DL Biocoenoses; PMC. Rosso Collection I.H. B-17a. Italy • 11 living and 33 dead colonies/large fragments, 1 dead colony including the base; off Acitrezza; sample AC/L, AC/1B; coordinates unknown; 50 and 110 m; 1980; I. Di Geronimo leg.; dredging; pre-Coralligenous and DL Biocoenoses; PMC. Rosso Collection I.H. B-17a1. Italy • 12 living and 315 dead colonies/fragments; Ionian Sea, Gulf of Noto; 36°41'45"–36°57'47"N, 15°8'35"–15°20'00"E; PS/81 cruise; samples 2C, 4X, 9D (living) and samples 2B, 2C, 2XA, 2XB, 4C, 4C1, 4X, 6D, 9C, 9D, 10C, 11E (dead); Jul. 1981; I. Di Geronimo leg.; dredging; DC and DL Biocoenoses; and 11 living colonies; Noto 1996 cruise; samples 8I, 10G, 10H; 77–82 m; 1996; E. Mollica leg.; dredging; DE and DL Biocoenoses; PMC. Rosso Collection I.H. B-17b. Italy • 18 dead colonies; Ionian Sea, Gulf of Catania; sample LCT69; 37°18'42"N, 15°14'24"E; 90 m; Jul. 1980; I. Di Geronimo leg.; dredging; DL Biocoenosis; PMC. Rosso Collection I.H. B-17c. Italy • 4 dead colonies; Ionian Sea, Gulf of Taranto, Amendolara Bank; samples 1D and 5D; 39°51'42"–39°52'54"N, 16°42'00"–16°43'24"E; 30–40 m; Jun. 1991; R. Sanfilippo leg.; dredging; DC Biocoenosis; PMC. Rosso Collection I.H. B-17g. Italy • 77 dead colonies and fragments; southern Tyrrhenian Sea, SW Ustica, Apollo Bank; 38°42'19"N, 13°7'58"E; 60 m; Jun. 1986, dredging; Laminaria rodriguezii Bornet, 1888 seagrass and associated DC Biocoenosis; PMC. Rosso Collection I.H. B-17d. Italy • 2 living colonies; Messina Strait; coordinates unknown; 65 m; 1990; S. Giacobbe leg.; dredging; no Biocoenosis information; PMC. Rosso Collection I.H. B-17g. France • 50 dead colonies; Iberian-Provençal Basin, Corsica, off Calvì; sample CL74; 42°47'31"N, 9°8'10"E; 150–110 m; G. Fredj leg.; dredging; DL Biocoenosis; PMC. Rosso Collection F.H. B-17e. Greece • 4 dead colonies, Aegean Sea, Lesvos Island, Agios Vasilios cave; samples AV1 and AV2; 38°58'8"N, 26°32'28"E; 30 m; V. Gerovasileiou leg.; scuba diving; GSO and GO Biocoenoses; PMC. Rosso Collection GR.H. B-17f.
Description
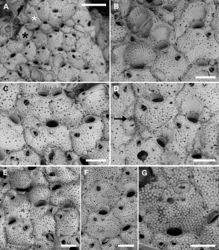
Colony erect, rigid, branched, with a limited number of relatively spaced-out bifurcations, a few cm long, raising from an encrusting basal portion (Fig. 10A), extending up to 3 mm around the main stem.
Branches cylindrical, often flattened at bifurcations (Fig. 10C, E), consisting of 9–16 longitudinal rows of zooids, alternating very regularly to simulate an helicoidal pattern; branch diameter 10–15 mm (exceptionally up to 20 mm), becoming thicker and stouter in older portions of the colony, near the encrusting base, owing to secondary calcification. Encrusting base unilaminar, multiserial (Fig. 10A), formed by autozooids similar to those of the erect branches (Fig. 10B) but with a greater number of oral spines (5–7, commonly six) and, subordinately, autozooids with occluded orifices (not functional) and/or kenozooids, often bearing an adventitious avicularium as those associated with autozooids. Interzooidal communications via basal pore-chambers in the encrusting portion (see Rosso et al. 2014[2]: fig. 3A) and via multiporous septula in the erect branches (Fig. 10C).
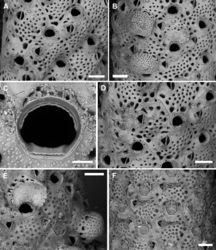
Autozooids rounded hexagonal to lozenge-shaped, 477–779 (661±93, N = 18) × 389–615 (493±68, N = 18) μm (mean L/W = 1.34), distinct, interzooidal boundaries marked by narrow, shallow, locally undulate grooves (Fig. 11A, B, D). Frontal shield nearly flat, finely granular, and pseudoporous; about 10 marginal areolae distinguishable from frontal pseudopores only in early ontogeny because larger, subcircular to elongate elliptical, 25–65 μm long; 19–26 subcircular pseudopores, 20–30 μm in diameter, placed centrally on the frontal (Fig. 11A, B, D). Transverse section of the branch showing thick frontal and vertical walls, converging towards the centre of the branch, forming wedge-like polypide cavities (Fig. 10E, F).
Primary orifice approximately semi-circular to horseshoe-shaped, 130–151 (143±6, N = 10) × 145–177 (161±10, N = 10) μm (mean OL/OW = 0.89; mean ZL/OL = 4.63), outlined by a thin and smooth raised rim (Fig. 11C); hinge-line smooth, straight to slightly concave with two short, blunt lateral condyles and a smooth, proximal shelf sloping outwards; distal margin of the orifice corrugated because of a deeply placed, drawstring-like, arched rim seemingly functioning as support for the closed operculum. Oral spines four, occasionally five, thin (base diameter 15–20 μm), relatively short (60–90 µm), placed distally, often detached (Fig. 11A, B, D).
Ascopore field a narrow, reniform to U-shaped rim of smooth gymnocystal calcification, 50–60 × 57–80 µm, placed 65–80 μm below the orifice, same level as the orifice and the adjacent frontal shield; ascopore opening transversely C-shaped, 40–63 × 5–12 μm, with a massive, upside-down mushroom-shaped tongue projecting from distal edge with radial spines (Fig. 11A, B).
A single, constant, large avicularium, 121–156 (142±9, N = 20) × 119–139 (130±7, N = 20) μm (mean AvL/AvW = 1.09), located laterally, on either side, at about half zooidal length (Figs 10B, C, 11A, B, D, F); crossbar complete; rostrum short, rounded triangular, channelled, directed laterally or less often distolaterally and slightly upward. Mandible 142–273 μm long, with a pointed, hooked tip, toothed at the level of the rostrum tip, lining proximally to the ascopore when open (Fig. 11F). Ovicell non-personate, subglobular, prominent, large, 250–327 (286±36, N = 4) × 384–430 (402±20, N = 4) μm (mean OvL/OvW = 0.71), formed by the distal autozooid, obscuring half of the zooidal orifice; calcification fabric similar to frontal shield but with larger and more prominent tubercles, and smaller (15–20 μm in diameter), more closely spaced pseudopores, seemingly radially aligned with rows separated by raised ridges; a discontinuous, peripheral row of larger pseudopores sometimes present (Figs 10C, 11B, E).
Ancestrula tatiform (Fig. 10A, B), oval (220 × 150 μm), gymnocyst concealed, cryptocyst smooth, narrowing distally; opesia oval (160 × 110 μm); only four, distal spines visible on the single ancestrula observed. Ancestrula budding two distolateral autozooids, and subsequently surrounded by two lateral and one proximal autozooids.
Older colony parts thickly calcified owing to secondary calcification progressively obliterating zooidal openings including orifices, ascopores and avicularia (Fig. 10D), making difficult the distinction between old autozooids and genuine kenozooids that probably also develop.
Remarks
First assigned to Eschara (Peach 1868[3]), Eschara verrucosa served as the type species of the genus Diporula Hincks, 1879 in which it has been included since then with the exception of Neviani (1896a[4], b[5]). Characters used to distinguish Diporula from Microporella included the “dendroid zoarium with cylindrical branches” and the morphology of the orifice described as “expanded above, contracted below, and slightly constricted by lateral projections (horseshoe-shaped)” (Hincks 1880[6]: 220; and similar description in Gautier 1962[7]: 176). However, both characters seem feeble to justify the distinction between the two genera. At least nine species of Microporella possess erect colony-growth, starting with a more or less developed encrusting portion as does Diporula. Also the shape of the orifice in Microporella species is highly variable (Di Martino et al. 2020a[8]), with several examples of species having orifices with the proximal margins narrower than the orifice maximum width [e.g., Microporella curta Almeida, Souza, Mengola & Vieira, 2017 from Brazil, Microporella franklini (Soule, Chaney & Morris, 2003) from California, the Mediterranean Microporella genisii (Audouin & Savigny, 1826), Microporella hastingsae Harmelin, Ostrovsky, Cáceres-Chamizo & Sanner, 2011 from the Red Sea, and the Arctic Microporella klugei Kukliński & Taylor, 2008].
Further differences between Microporella and Diporula were highlighted by Hayward and Ryland (1999[9]: 292, 312), including interzooidal communications via basal pore-chambers in the former genus and multiporous septula in the latter, and the presence of pseudopores in the ovicells of Diporula. However, multiporous septula were observed, for example, in Microporella ordo (see Di Martino et al. 2020a[8]: fig. 7D), and basal pore-chambers were observed in the encrusting portions of M. verrucosa, while pseudopores occur in the ooecium of many Microporella species including the type M. ciliata (see Kukliński and Taylor 2008[10]: fig. 1c). A further presumed difference relates to the ooecium porosity, with Diporula reported as having a fully perforated endooecium and Microporella species usually described as having only pits in the endooecium (Harmelin et al. 2011[11]: 2; Ostrovsky 2013[12]: figs 2.43B–D, 2.44A). However, pores clearly perforate the endooecium also in Microporella as seen in broken ooecia of M. ichnusae sp. nov. (Fig. 6B)
Based on these observations, here we propose Diporula as junior synonym of Microporella and resurrect the combination Microporella verrucosa first proposed by Neviani (1896a[4], b[5]). Specimens of a second species of Diporula, D. coronula Ortmann, 1890 need re-examination. Based on the original description and illustration (Ortmann 1890[13]: 39, pl. 3, fig. 7), this species has a lepralioid orifice with condyles at about one-third of the orifice length, a single avicularium with spathulate mandible, and up to two frontal foramina, characters reminiscent of other cheilostome genera such as, for example, Poricella Canu, 1904.
Specimens originally described as Eschara lunaris Waters, 1878, from Pleistocene sediment of eastern Sicily and synonymised with M. verrucosa by Hincks (1880)[6] need to be re-examined as well to confirm their conspecificity, but this is out of the scope of the present paper.
The rugose appearance observed by Peach (1868)[3] and Hincks (1880)[6], which inspired the species name, was not observed in our material, although secondary calcification is always very common in older parts of the colony. Intramural budding is rare and restricted to avicularia, while branch regeneration is common, apparently following breakage as indicated by broken autozooids with sharp edges. We also observed zooids with reverse polarity, sometimes budded from old stems with autozooids obliterated by secondary calcification. However, in these instances few whorls of autozooids usually develop from the regeneration surface, with only few tips appearing actively growing. Colony fragments longer than 2 cm can appear twisted, a morphology observed in some cyclostomes (Harmelin 1976[14]) and other erect cheilostomes from the Gulf of Noto and the Ciclopi MPA area (Rosso 1989[15]). This twisted branch morphology and the ability to regenerate after breakage might represent the adaptation of this species to colonize soft sediment bottoms. Basal, encrusting colony portions are relatively common in our samples and show that the ancestrulae settled on clasts ranging from a few mm to 1–2 cm in size. The ability of this species to encrust small particles, in addition to large substrates in rocky habitats, was suggested by Gautier (1962)[7] after finding only colony fragments in dredges from sandy-muddy bottoms.
The diagnostic characters of this species seem constant in the Mediterranean specimens, except for the size of the ascopore related to the development of the distal tongue sometimes leaving only a fissure-like opening. Paired avicularia were observed only in one autozooid (Fig. 11E). Higher variability is observed when comparing the Mediterranean specimens with those from the Atlantic (e.g., Hayward and Ryland 1999[9]: 302, figs 138C, D, 139A, B; unpublished SEM images provided by P.D. Taylor from Mauritania and Madeira) related to the ascopore shape, the size of the spines, and the distribution and size of pseudopores on the frontal shield, suggesting the existence of a species complex.
Distribution and ecology
Microporella verrucosa is a warm-temperate species with Atlanto-Mediterranean distribution. In the Atlantic, it has been reported from West Africa to the southwest of the British Isles (Hayward and Ryland 1999[9]); in the Mediterranean, it occurs preferentially in mid- and outer-shelf habitats below 50–60 m depth, with an optimum at 70–120 m (Gautier 1962[7]; Zabala 1986[16]; Rosso 1989[15], 1996a[17]; Rosso and Di Martino 2016[18]), but it was also observed at shallower depths (20 m) in a shadowed open cave in Catalonia (André et al. 2014[1]). It is associated with shadowed rocky habitats, including the Coralligenous and Semi-Dark and Dark Cave Biocoenoses, and detritic habitats, such as the Coastal Detritic and the Offshore Detritic Biocoenoses (Table 1; Gautier 1962[7]; Harmelin 1969[19], 1976[14]; Zabala 1986[16]; Rosso 1989[15], 1996a[17], 1996b[20]; Di Geronimo et al. 1990[21]; Madurell et al. 2013[22]; Rosso et al. 2014[2], 2019b[23]; Gerovasileiou and Rosso 2016[24]). However, it is never very common or dominant at sample or habitat scale, occurring only with a few colonies per sample and/or in one out of four or five sampling stations (Table 1; see also Harmelin 1976[14]: tables 1, 3).
| Sea/ Locality | Sample | Depth | Biocoenosis | appendiculata | bicollaris sp. nov. | ciliata | ichnusae sp. nov. | modesta | pachyspina sp. nov. | sp. A | verrucosa |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ionian/ Gulf of Noto | PS/81 CR1 | 45 | DC | 27(8) | 16(12) | ||||||
| PS/81 2B | 65 | DC | (2) | ||||||||
| PS/81 2C | 83–74 | DC | 1(44) | ||||||||
| PS/81 2XA | 128 | DL | (4) | ||||||||
| PS/81 2XB | 120 | DL | (1) | (28) | |||||||
| PS/81 4C | 95–86 | DL | (1) | (32) | |||||||
| PS/81 4C1 | 89–84 | DL | (44) | ||||||||
| PS/81 4X | 102–93 | DL | (1) | 1(33) | |||||||
| PS/81 6D | 98–96 | DL | (87) | ||||||||
| PS/81 9B | 44 | DC | (1) | (1) | |||||||
| PS/81 9C | 60 | DC | (1) | (3) | |||||||
| PS/81 9D | 78 | DC | (12) | 10(24) | |||||||
| PS/81 10C | 60 | DC | (1) | (1) | (1) | ||||||
| PS/81 11E | 98 | DL | (2) | (13) | |||||||
| N/96 3C | 20 | C | 11 | ||||||||
| N/96 5E | 40 | C-DC | 1 | ||||||||
| N/96 6C | 45 | VTC | 2 | ||||||||
| N/96 7E | 35 | C | 12 | ||||||||
| N/96 8I | 77 | DE | 1 | ||||||||
| N/96 9E | 50 | DC | 1 | ||||||||
| N/96 10G | 82 | DE | 1 | 6 | |||||||
| N/96 10H | 80 | DE-DL | 4 | ||||||||
| N/96 10 I | 107 | DL | (1) | ||||||||
| N/96 WP | 90 | DL | (1) | (24) | |||||||
| Ionian/ Plemmirio caves | MZ1 | ≈23 | GSO | (1) | |||||||
| GR E | ≈19 | C | 3(1) | ||||||||
| Ionian/ Gulf of Catania | Cast. beac. | 0 | na | 15 | |||||||
| LCT69 | 90 | DL | (2) | (18) | |||||||
| Ognina | 4 | plastic | 2 | ||||||||
| CI 2G | 87.5 | DE-DL | (4) | 1(3) | |||||||
| CI 3H | 71 | DC | (1) | ||||||||
| CI 4E | 52 | DC | 1 | (1) | |||||||
| CI 6H | 75 | DC? | 1(2) | ||||||||
| CI 8F | 79 | DC | (1) | ||||||||
| CI 8H | 92.5 | DE-DL | (1) | ||||||||
| CI 8I | 95 | DE-DL | (3) | (1) | |||||||
| CI 9G | 63 | DC | (10) | (30)f | |||||||
| CI 10G | 85 | DC-DE | (1) | ||||||||
| CI 12E | 62 | DC | (7) | (2) | |||||||
| CI 12F | 70 | DC | (1) | (2) | |||||||
| CI 12G | 83 | DE-DL | (5) | (1) | |||||||
| CI 13H | 105 | DL | 10(32) f | ||||||||
| CI 14G | 90 | DL | 1 | (1) | |||||||
| AC/L | 50 | C | (16) | ||||||||
| AC/1B | 110 | DL | 11(18) f | ||||||||
| SM1Z25 | 25 | IA | 1 | ||||||||
| Ionian | Messina Strait | 65 | no data | 2 | |||||||
| Ionian/ Gulf of Taranto | AM 1D | 30–40 | DC | 1 | (3) | ||||||
| AM 5D | 40 | DC | 1 | (1) | |||||||
| PCI 10 | 5–15 | C | 2(1) | ||||||||
| Sicily Strait/ Pelagian Island | Ta I cave | 10–20 | C-GSO | 1(1) | |||||||
| Ma cave | 15 | GSO | 1 | ||||||||
| Sc cave | 10 | GSO | 2(2) | ||||||||
| Sicily Strait/ Egadi Island | ECE 5 | 8 | IA; IA-HP | ca.100 | 3 | ||||||
| EBE/EBI | 19 | 15 | |||||||||
| Tyrrhenian | Palinuro c. | 46 | GO | (1) | |||||||
| Ustica Isl. | 60 | C | 6(2) | (77) | |||||||
| Iberian-Provençal basin/Sardinia, Capo Caccia, and Asinara | Falco 1 | 7 | GSO | 2 | |||||||
| Falco 2 | 4 | 3 | |||||||||
| Bisbe 1 | 8 | 2 | 1 | ||||||||
| Bisbe 2 | 8 | 1 | 4 | ||||||||
| Galatea 1 | 8 | 1 | |||||||||
| Galatea 2 | 6 | 1 | |||||||||
| PSE/PSI | 5–15 | IA | 7 | ||||||||
| W Corsica | CL 74 | 150–110 | DL | (11) | (50) | ||||||
| Adriatic | Bari cn. 1B1 | 280 | CB | (1) | |||||||
| Aegean/ Lesvos Island | AV1 | 30 | GSO | (1) | (1) | ||||||
| AV2 | 30 | GO | (3) |
Taxon Treatment
- Martino, E; Rosso, A; 2021: Seek and ye shall find: new species and new records of Microporella (Bryozoa, Cheilostomatida) in the Mediterranean ZooKeys, 1053: 1-42. doi
Images
|
Other References
- ↑ 1.0 1.1 André J, Corolla B, Lanza B, Rochefort G (2014) Bryozoaires d’Europe. Les carnets du plongeur.Edition Neptune Plonges, Audry & Schaffer, Marseille, 255 pp.
- ↑ 2.0 2.1 2.2 Rosso A, Sanfilippo R, Sciuto F (2014) Open shelf soft bottom bryozoan communities from the Ciclopi Marine Protected Area (E Sicily, Mediterranean). In: Rosso A Wyse Jackson P Porter J (Eds) Bryozoan Studies 2013. Proceedings of the 16th Conference of the International Bryozoological Association, Catania, Italy.Studi Trentini di Scienze Naturali94: 195–207.
- ↑ 3.0 3.1 Peach C (1868) On a new British Eschara, and the occurrence, in Cornwall, of Sphenotrochus Wrightii, of Gosse.Journal of the Royal Institution of Cornwall3: 116–117.
- ↑ 4.0 4.1 Neviani A (1896a) Briozoi fossili della Farnesina e di Monte Mario presso Roma.Paleontographia Italica1: 77–140.
- ↑ 5.0 5.1 Neviani A (1896b) Briozoi postpliocenici di Spilinga (Calabria). Atti Accademia Gioenia di Scienze Naturali in Catania 9, ser.4: 1–66.
- ↑ 6.0 6.1 6.2 Hincks T (1880) A history of the British Marine Polyzoa.Van Voorst, London, 601 pp. [83 pls.] https://www.biodiversitylibrary.org/page/23373713
- ↑ 7.0 7.1 7.2 7.3 Gautier Y (1962) Recherches ecologiques sur les Bryozoaires Chilostomes en Méditerranée occidentale.Recueillis des Travaux de la Station Marine d’Endoume38: 1–435.
- ↑ 8.0 8.1 Di Martino E, Taylor P, Gordon D (2020a) Erect bifoliate species of Microporella (BryozoaCheilostomata), fossil and modern.European Journal of Taxonomy678: 1–31. https://doi.org/10.5852/ejt.2020.678
- ↑ 9.0 9.1 9.2 Hayward P, Ryland J (1999) Cheilostomatous Bryozoa. Part 2. Hippothoidea – Celleporoidea. Barnes RSK, Crothers JH (Eds) Synopses of the British Fauna (New Series) Field Studies Council, Shrewsbury 14: 1–416.
- ↑ Kukliński P, Taylor P (2008) Arctic species of the cheilostome bryozoan Microporella, with a redescription of the type species.Journal of Natural History42(27–28): 1893–1906. https://doi.org/10.1080/00222930802126904
- ↑ Harmelin J, Ostrovsky A, Cáceres-Chamizo J, Sanner J (2011) Bryodiversity in the tropics: taxonomy of Microporella species (Bryozoa, Cheilostomata) with personate maternal zooids from Indian Ocean, Red Sea and southeast Mediterranean.Zootaxa2798: 1–30. https://doi.org/10.11646/zootaxa.2798.1.1
- ↑ Ostrovsky A (2013) Evolution of sexual reproduction in marine invertebrates. Example of Gymnolaemate bryozoans.Springer, Dordrecht, 356 pp. https://doi.org/10.1007/978-94-007-7146-8
- ↑ Ortmann A (1890) Die Japanische Bryozoenfauna. Bericht über die von Herrn Dr.L.Döderlein in Jahre 1880–81, gemachten Sammlungen.Archiv für Naturgeschichte56: 1–74.
- ↑ 14.0 14.1 14.2 Harmelin J (1976) Le sous-ordre des Tubuliporina (Bryozoaires Cyclostomes) en Méditerranée. Écologie et systématique.Mémoires de l’Institut océanographique10: 1–326.
- ↑ 15.0 15.1 15.2 Rosso A (1989) Contributo alla conoscenza di alcuni popolamenti, tanatocenosi e tafocenosi a briozoi di alcuni fondi mobili circalitorali. PhD thesis, University of Messina.
- ↑ 16.0 16.1 Zabala M (1986) Fauna dels bryozous dels Països Catalans. Barcelona.Institut D’estudis Catalans Secció de Ciències84: 1–836.
- ↑ 17.0 17.1 Rosso A (1996a) Popolamenti e tanatocenosi a Briozoi di fondi mobili circalitorali del Golfo di Noto (Sicilia SE). Naturalista Siciliano (Serie 4) 20(3–4): 189–225.
- ↑ Rosso A, Di Martino E (2016) Bryozoan diversity in the Mediterranean Sea: an up-date.Mediterranean Marine Science17(2): 567–607. https://doi.org/10.12681/mms.1706
- ↑ Harmelin J (1969) Bryozoaires des grottes sous-marines obscures de la région Marseillaise: faunistique et écologie.Téthys1: 793–806.
- ↑ Rosso A (1996b) Valutazione della biodiversità in Mediterraneo: l’esempio dei popolamenti a briozoi della Biocenosi del Detritico Costiero.Biologia Marina Mediterranea3(1): 58–65.
- ↑ Di Geronimo I, Giacobbe S, Rosso A, Sanfilippo R (1990) Popolamenti e tanatocenosi del Banco Apollo (Ustica, Mar Tirreno meridionale).Bollettino del Museo Regionale di Scienze Naturali Torino vol. spec.: 729–. $6
- ↑ Madurell T, Zabala M, Dominguez-Carrió C, Gili J (2013) Bryozoan faunal composition and community structure from the continental shelf off Cap de Creus (Northwestern Mediterranean).Journal of Sea Research83: 123–126. https://doi.org/10.1016/j.seares.2013.04.013
- ↑ Rosso A, Gerovasileiou V, Sanfilippo R, Guido A (2019b) Bryozoans assemblages from two submarine caves in the Aegean Sea (Eastern Mediterranean).Marine Biodiversity49(2): 707–726. https://doi.org/10.1007/s12526-018-0846-0
- ↑ Gerovasileiou V, Rosso A (2016) Marine Bryozoa of Greece: an annotated checklist. Biodiversity Data Journal 4: e10672. https://doi.org/10.3897/BDJ.4.e10672