Lactuca hazaranensis
Contents
- 1 Taxonavigation
- 2 Name
- 3 Diagnosis
- 4 Holotype
- 5 Description
- 6 Distribution and habitat
- 7 Etymology
- 8 Morphological affinities and delimitation
- 9 Preliminary conservation status
- 10 Transversally constricted achenes of Lactuca hazaranensis aiding myrmecochory?
- 11 Original Description
- 12 Other References
- 13 Images
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Kilian2012PhytoKeys11, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Kilian2012PhytoKeys11">{{Citation See also the citation download page at the journal. |
Ordo: Asterales
Familia: Asteraceae
Genus: Lactuca
Name
Lactuca hazaranensis Djavadi & N. Kilian sp. nov. – Wikispecies link – IPNI link – Pensoft Profile
Diagnosis
Habitually similar to Lactuca rosularis but clearly distinguished from it by the rosette leaves being undivided (instead of lyrately to irregularly pinnatifid to pinnatisect), the peduncles being distinct, c. 0.3–0.7(–1) cm long (instead of indistinct, 0–0.2 cm long), the achenes having a 0.3–0.5 mm (instead of (1.1–)3–5.3 mm) long beak and the achene body being transversally constricted and sectioned in the distal quarter into an embryo-containing proximal and a solid distal segment (instead of being unconstricted and unsectioned).
Holotype
Iran. Kerman: Rayen, near Rayen falls [c. 29°33'N, 57°18'E], 2850 m, 10 Aug 2010, M. Eskandari & A. Torabi(IRAN 55199; photo at B).
Description
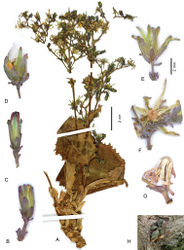
Perennial rosulate herb (Fig. 2A), with a taproot(?) and a woody caudex covered by the marcescent remains of old leaf bases. Stem one or a few per leaf rosette, erect, (2–)10–18 cm tall, branched already in lower half. Rosette leaves obovate to spatulate, (2–)5–11 × (1–)2–5 cm, somewhat glaucous; base semiamplexicaul, margin densely, coarsely and ± irregularly dentate-denticulate, apex subacute to acute. Lower and middle stem leaves spatulate to lanceolate, with auriculately clasping base, smaller, otherwise similar to rosette leaves; upper stem leaves distinctly smaller than lower and middle ones, lanceolate to ovate, with conspicuously auriculately clasping base, margin usually entire, apex acute to acuminate; uppermost stem leaves bractlike. Synflorescence (Fig. 2A) of a stem corymbosely paniculiform, with some to many capitula, axes wiry; peduncles c. 0.3–0.7(–1) cm long, capillaceous. Capitula with 7–14 flowers. Involucre (Fig. 2B–G) narrowly cylindric at anthesis, 6–7 mm long, not elongating during maturation; outer phyllaries imbricate, outermost ovate to narrowly ovate, 1.5–3 mm long, similar to the bracts on the peduncle, following ones gradually longer and ovate-lanceolate to lanceolate, the longest up to c. 1/2 as long as inner ones; inner phyllaries linear-lanceolate, 6–9, ± in one row, somewhat unequal in length, with ± narrow scarious margin. Receptacle flat to slightly convex, naked. Flowers with corolla yellow, ligule 5.5–6.5 mm long, tube shorter than ligule; anther tube without appendages 1.6–2 mm, basal appendages c. 0.4 mm, apical appendages 0.4 mm long; style arms 1.6–1.8 mm long. Achenes (Fig. 3A) homomorphic, including beak 3.2–3.6 mm long, corpus 2.9–3.2 mm long, up to 0.8–1.1 mm in diam., ellipsoidal, compressed, with a transversal constriction of 0.4–0.8 mm diam. in the distal 1/3–1/4, distal segment c. 1.5 × 1.5 mm, contracted into a stout, easily detachable beak c. 0.3–0.5 mm long; achene body (Fig. 3F–I) apart from the two lateral ribs with 1 similarly strong median rib on either side, rarely dorsally with 2 equally strong ribs, secondary ribs missing or rarely 1–2 per side; achene surface faintly transversally wrinkled, proximal segment brown, distal segment and beak yellowish, ribs straw-coloured to yellowish in distal segment; proximal segment containing the whitish embryo (Fig. 3H–I), distal segment containing yellowish tissue (Fig. 3H). Pappus simple, without an outer series of minute hairs, setae thin, white, 3–3.5 mm long, easily detachable. – Flowering and fruiting: June to August.
Distribution and habitat
The type collection of Lactuca hazaranensis comes from the northeastern foot of Mt Hezar, which rises to 4465 m elevation, and has been collected in the vicinity of the Rayen falls, at an altitude of 2850 m, in rock crevices. A second collection, with mature achenes of the precisely the same variant, was traced in the Berlin herbarium and had been made by J. Bornmüller in 1892 some 50 km further NW on rocks at an altitude of 3700 m on Mt Jupar (c. 29°55.8'N, 57°11.5'E; spelled “Khu-i-Dschupar” by Bornmüller, see also Freitag and Kuhle 1980[1]), which is also situated in the Hazaran or Hezar-Lalezar mountain range (Fig. 4). Bornmüller (1939[2]: 224), who determined this collection as Lactuca rosularis, characterised it as very rare on Mt Jupar, having only traced three tiny individuals (all preserved on the single sheet at B). The Hazaran or Hezar-Lalezar mountain range, which is mainly composed of limestone, is the highest mountain range in southeastern Iran and known as a local centre of endemism (Noroozi et al. 2010[3]). Additional specimen seen: Iran. Kerman: in rupibus summi jugii m. Kuh-i-Dschupar [Kuh-i-Jupar, c. 29°55.8'N, 57°11.5'E], 3700 m, 13 Jun 1892, J. Bornmüller 4119 (B).
Etymology
Lactuca hazaranensis is named after its provenance, the Hazaran mountain massif in the Iranian province of Kerman, which is a southeastern outlier of the Zagros mountain range and reaches a maximum elevation of about 4500 m in the peak Kuh-e Hazar.
Morphological affinities and delimitation
. Morphological comparison revealed that the new species is most similar to both Lactuca rosularis and Lactuca denaensis. The three species are perennial rosette herbs of montane to high-montane environments, which are most likely closely related to each other, and considered here as the Lactuca rosularis group. They are all rare, being known from few collections only, and are endemic or almost so to the Iranian Highlands (Fig. 4). They share the rosulate habit with a woody caudex, the glaucous leaves, small yellow-flowered capitula and the principally same achene morphology. The differences between these three species are summarised in Table 1. {| class="wikitable" ; style="width: 100%"
|+ Table 1. Morphological differences between Lactuca hazaranensis and its relatives Lactuca rosularis and Lactuca denaensis, as well as between the latter and Cicerbita polyclada. Based on the material studied. – The investigated material is listed in the Appendix.
|-
! Features !! Lactuca hazaranensis !! Lactuca rosularis !! Lactuca denaensis !! Cicerbita polyclada
|-
| Rosette leaves: division || undivided || lyrately to irregularly pinnatifid to pinnatisect with large terminal lobe || undivided || undivided, towards base sometimes shallowly pinnately divided
|-
| Rosette leaves: margin || coarsely and ± irregularly dentate and denticulate || irregularly dentate and denticulate || ± densely dentate and denticulate || subentire to dentate and denticulate
|-
| Branching of stem || with dominating main axis, corymbosely paniculiform || with dominating main axis, paniculiform to corymbosely paniculiform || subacaulescent,
with dominating main stem only few cm long or branched from base, corymbosely paniculiform || ± without dominating main axis, repeatedly divaricately branched from base
|-
| Branches || slender to capillaceous || ± slender (to capillaceous) || slender to capillaceous || conspicuously inflated
|-
| Penduncles, length [cm], shape || c. 0.3–0.7(–1), capillaceous || 0–0.2, capillaceous if developed || 0.4–0.7, capillaceous || 1–3.5, inflated if well developed
|-
| Cauline leaves || usually present, gradually reduced to bracts || basally present, soon reduced to bracts || usually absent, reduced to bracts || absent
|-
| Involucre, length [mm] || 6–7 || 6–9(–10) || 10–12 || 7–9
|-
| Corolla, colour || yellow || yellow || yellow || blue
|-
| Achene, length [mm] || 3.2–3.6 || (3.8–)6–8 || 4.8–5.7 || 3.4–5.2
|-
| Achene: corpus, length [mm] || 2.9–3.2 || 2.2–3 || 4–5 || 3–4.2
|-
| Achene: ribs || 4(–5), strongly prominent || 4, strongly prominent || 4(–5), strongly prominent || 5, subprominent to subdistinct
|-
| Achene: beak, length [mm] || 0.3–0.5 || (1.1–)3–5.3 || 0.4–0.9(–2) || 0.5–1
|-
| Achene: transversal constriction || present || absent || absent || absent
|-
| Pappus, length [mm] || 3–3.5 || 2.5–3 || 5–6 || 3–4
|-
| Pappus: outer ring of minute hairs || absent || absent || absent || present
|}
Preliminary conservation status
Lactuca hazaranensis is known only from two localities c. 50 km apart, which are not in protected areas. The species seems to be rare, but it has to be taken into account that it is rather inconspicuous in its rocky environment. Members of the tribe are among the most favoured food of livestock, grazed wherever in reach and are therefore particularly threatened by overgrazing. Lactuca hazaranensis must currently be assessed as Data Deficient (IUCN 2001[4]), but since the status Endangered seems not unlikely, an assessment of its populations in the field would be desirable.
Transversally constricted achenes of Lactuca hazaranensis aiding myrmecochory?
The shape of the achenes of Lactuca hazaranensis with the transversal constriction in the distal third (Fig. 3A, F–H) is curious. The fact that all achenes of all fruiting heads in two collections from different localities and centuries invariably show the same morphology, rules out the possibility that this achene variant represents a teratogenic manifestation.
Conspicuous transversal constrictions are, as far as we know, a very rare phenomenon in Asteraceae fruits. The present case is parallelled, however, by a few Pulicaria species of the Horn of Africa and southern Yemen (Wagenitz and Gamal-Eldin 1983[5] under Sclerostephane; Kilian 1999[6]), which are likely not all closely related to each other (Englund et al. 2009[7]) in contrast to what was thought initially. The possible function of these constrictions in the Pulicaria species is unknown.
In contrast to the cases in Pulicaria, where the constrictions chiefly affect the pericarp (Kilian 1999[6]: fig. 2c, 3a, 4a, 5c), the constriction in Lactuca hazaranensis incompletely divides the achene into two segments (Fig. 3H). The large proximal segment contains the whitish embryo (Fig. 3I), the small distal segment is solid and of a yellowish tissue, which is partly identical partly contiguous with and therefore apparently derived from the intercostal yellowish pericarp tissue. The tissue of the embryo and the tissues of the distal segments are somewhat spatially separated from each other (Fig. 3H).
Conspicuous segmentation of the achene, although not precisely by a transversal constriction, is otherwise known from the probably unique case of the bispecific genus Urospermum (Cichorieae, Hypochaeridinae): the achenes of this genus consist of a proximal, compressed segment, which contains the embryo, and a larger, inflated distal segment tapering into the beak (for images, see Urospermum picroides in ICN 2011[8]). In contrast to our case, in Urospermum both segments are separated from each other by a transversal wall and the distal segment is hollow (Lack and Leuenberger 1979[9]).
A morphological transition towards a segmentation might perhaps be an achene with a cavity below the beak as it can be observed, e.g. in Cicerbita polyclada (Fig. 3D–E). Within subtribe Lactucinae, the achenes of Cephalorrhynchus polycladus (Boiss.) Kirp., not to be confused with the habitually similar Cicerbita polyclada, might represent an even stronger morphological transition (pers. com. A. Sennikov, Feb 2012); the presumably empty apical achene portion below the beak is, as stated by Kirpicznikov (1964[10]: 351 + t. 20, fig. 8), somewhat narrower and separated by a very slight (non-waisted) constriction proximally.
Being shortly beaked and provided with a pappus, the achenes of Lactuca hazaranensis appear principally suited for wind dispersal (anemochory). The entire beak is, however, detachable at its base from the distal segment of the achene by slightest pressure and also the pappus setae are very easily detachable and are thus not functional for dispersal by wind. Finally, the solid distal segment brings additional weight and thus impedes wind dispersal.
Light microscopic histochemical analysis, using Sudan staining, of the yellow tissue of the distal segment of Lactuca hazaranensis revealed abundant presence of lipid drops, which were similarly found also in the embryo. Lipids are well known in the embryo of Lactuca as a major component of the reserves for the germination, accounting for 33 % dry weight of the achene in lettuce (Lactuca sativa) (Paulsen and Srivastava 1968[11]; Srivastava and Paulsen 1968[12]; Halmer et al. 1978[13]), and for 35 % in the oilseed lettuce cultivar of Lactuca sativa with particularly large fruits grown in Egypt as a source for cooking oil (Křístková et al. 2008[14]).
Their spatial separation from the embryo makes it unlikely that the lipid reserves of the distal segment are related to the germination. A potential function for the dispersal of the achene seems more probable, in particular in connection with the easy detachment of the achene beak. The detachment of the beak has not only a potentially atelechorous effect but the rupture also exposes the lipid reserves of the distal segment. The distal segment could thus perhaps be an “elaiosome”, a structure developing from seed or fruit tissue and aiding diaspore dispersal by ants (myrmecochory) in that it both attracts and rewards them (Bresinsky 1963[15]; Lengyel et al. 2010[16]). Usually, ants carry the diaspores into their nest, consume the lipid-rich tissue or feed it to their larvae and finally dump the diaspore in or outside their nest. Elaiosomes have convergently developed in seed plants many times, being known from 77 families and 334 genera (Lengyel et al. 2010[16]). They are also long known from the Asteraceae (Sernander 1906[17]; Nesom 1981[18]), having been reported from many Cardueae species and also from five other tribes (Anthemideae, Arctotideae, Calenduleae, Heliantheae, Senecioneae), but not so far from the Cichorieae (Lengyel et al. 2010[16]).
Usually, elaiosomes develop in Asteraceae at the base of the achenes from tissue separating the achene from the receptacle. In this way, the elaiosome is separated from the embryo by the indurate pericarp, which hinders the ants to get access to the embryo in the interior. The development of an elaiosome at the apex of the achenes and inside the pericarp appears in this context much less favourable. Apart from the presumably higher cost of this solution, the constriction only incompletely locks off the lipid reserves from the embryo, with the risk of its damage. In case the hypothesis of the myrmecochorous property of the distal achene segment of Lactuca hazaranensis is confirmed, e.g. by an experimental approach such as exemplified by Nesom (1981)[18], it certainly would make an interesting case, considering both the chasmophytic growth of the plants and their apparent rarity.
The unparallelled and, so far as we know, transition-free occurrence of the transversally constricted achenes in the Lactuca rosularis group, is, independently of its potential function, a particularly striking evidence for a considerable developmental plasticity in achene features in the Lactucinae.
Original Description
- Kilian, N; Djavadi, S; Eskandari, M; 2012: Two new mountainous species of Lactuca (Cichorieae, Asteraceae) from Iran, one presenting a new, possibly myrmecochorous achene variant PhytoKeys, 11: 61-77. doi
Other References
- ↑ Freitag H, Kuhle M (1980) A plant list from the Kuh-e-Jupar (S. E. Iran), with some ecological remarks. Willdenowia 10: 161–169. http://www.jstor.org/stable/3996141
- ↑ Bornmüller J (1939) Iter Persico-turcicum 1892–1893. Beiträge zur Flora von Persien, Babylonien, Assyrien, Arabien. Fortsetzung III. Beihefte Botanisches Centralblatt, B, 60: 181-228.
- ↑ Noroozi J, Yousef A, Nordenstam B (2010) A new annual species of Senecio (Compositae-Senecioneae) from subnival zone of southern Iran with comments on phytogeographical aspects of the area. Compositae Newsletter 48: 43-62.
- ↑ IUCN Species Survival Commision (2001) IUCN Red List Categories: Version 3.1. IUCN, Gland and Cambridge.
- ↑ Wagenitz G, Gamal-Eldin E (1983) Die Gattung Sclerostephane Chiov. (Compositae, Inuleae). Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 104: 91-113.
- ↑ 6.0 6.1 Kilian N (1999) Studies in the Compositae of the Arabian Peninsula and Socotra – 1. Pulicaria gamaleldinae sp. nova (Inuleae) bridges the gap between Pulicaria and former Sclerostephane (now P. sect. Sclerostephane). Willdenowia 29: 167–185. http://www.ingentaconnect.com/content/bgbm/will/1999/00000029/F0020001/art00016
- ↑ Englund M, Pornpongrungrueng P, Gustafsson M, Anderberg A (2009) Phylogenetic relationships and generic delimitation in Inuleae subtribe Inulinae (Asteraceae) based on ITS and cpDNA sequence data. Cladistics 25: 319-352. doi: 10.1111/j.1096-0031.2009.00256.x
- ↑ ICN (2009+) Hand R Kilian N Raab-Straube E(Eds) Urospermum picroides. In: International Cichorieae Network: Cichorieae Portal. http://wp6-cichorieae.e-taxonomy.eu/portal/?q=cdm_dataportal/taxon/da1ef992-e589-48cb-9670-fd76ef8cf4fd/images
- ↑ Lack H, Leuenberger B (1979) Pollen and taxonomy of Urospermum (Asteraceae, Lactuceae). Pollen and Spores 21: 415-425.
- ↑ Kirpicznikov M (1964) Subtribe 5. Lactucinae [p.p.]. In: Bobrov E Tzvelev N (Eds). , Flora SSSR 29. Moskva et Leningrad, Nauka: 237-375.
- ↑ Paulson R, Srivastava L (1968) The fine structure of the embryo of Lactuca sativa. I. Dry embryo. Canadian Journal of Botany 46: 1437-1445. doi: 10.1139/b68-195
- ↑ Srivastava L, Paulson R (1968) The fine structure of the embryo of Lactuca sativa. II. Changes during germination. Canadian Journal of Botany 46: 1447-1453. doi: 10.1139/b68-196
- ↑ Halmer P, Bewley J, Thope T (1978): Degradation of the endosperm cell walls of Lactuca sativa L., cv. Grand Rapids. Timing of mobilisation of soluble sugars, lipid and phytate. Planta 129: 1-8. doi: 10.1007/BF00390802
- ↑ Křístková E, Doležalová I, Lebeda A, Vinter V, Novotná A (2008) Description of morphological characters of lettuce (Lactuca sativa L.) genetic resources. Horticultural Science(Prague) 35: 113–129. http://www.agriculturejournals.cz/web/hortsci.htm?volume=35&firstPage=113&type=publishedArticle
- ↑ Bresinsky A (1963) Bau, Entwicklungsgeschichte und Inhaltsstoffe der Elaiosomen. Studien zur myrmekochoren Verbreitung von Samen und Früchten. Bibliotheca Botanica 126: 1-54.
- ↑ 16.0 16.1 16.2 Lengyel S, Gove A, Latimer A, Majer J, Dunn R (2010) Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: a global survey. Perspectectives in Plant Ecolology, Evolution and Systematics 12: 43–55. http://dx.doi.org/10.1016/j.ppees.2009.08.001
- ↑ Sernander R (1906) Entwurf einer Monographie der europäischen Myrmekochoren. Kongliga Svenska Vetenskaps Academiens Handlingar 41: 1-410.
- ↑ 18.0 18.1 Nesom G (1981) Ant dispersal in Wedelia hispida HBK (Heliantheae: Compositae). Southwestern Naturalist 26: 5–12. http://www.jstor.org/stable/3671323 doi: 10.2307/3671323
Images
|
- ↑ Kirpicznikov M (1964) Subtribe 5. Lactucinae [p.p.]. In: Bobrov E Tzvelev N (Eds). , Flora SSSR 29. Moskva et Leningrad, Nauka: 237-375.
- ↑ Tuisl G (1977) Lactuca. In: Rechinger K (Ed). , Flora Iranica 122. Graz, Akademische Druck- und Verlagsanstalt: 185-196.
- ↑ Hijmans R (2011) DIVA-GIS, ver. 7.4. http://diva-gis.org/
- ↑ CGIAR-CSI [Consortium of Spatial Information] (2004) NASA Shuttle Radar Topographic Mission (SRTM) 90 m digital elevation data (DEMs). http://srtm.sci.cgiar.org/

![Figure 4. Distribution of Lactuca hazaranensis (circles) and the related species Lactuca rosularis (squares) and Lactuca denaensis (rhomb, actucal position as indicated by arrow), as well as of Cicerbita polyclada (triangles). – Georeferenced map based on the known collections (see Appendix and supplemented by collection cited in Kirpicznikov 1964[1] and Tuisl 1977[2]) and generated with DIVA-GIS (Hijmans 2011[3]) using an adaptation of the SRTM 90 m digital elevation data (CGIAR-CSI 2004[4]).](https://species-id.net/o/thumb.php?f=PhytoKeys-011-061-g004.jpg&width=250)