Microporella bicollaris
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Martino2021ZooKeys1053, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Martino2021ZooKeys1053">{{Citation See also the citation download page at the journal. |
Ordo: Cheilostomatida
Familia: Microporellidae
Genus: Microporella
Name
Microporella bicollaris Martino & Rosso, 2021 sp. nov. – Wikispecies link – ZooBank link – Pensoft Profile
- Microporella sp. C Rosso et al. 2013a[1]: table 17.1; Rosso et al. 2013b[2]: table 1.
Type material
Holotype: Italy • The largest of 2 living colonies on the basal part of a thallus of Halimeda tuna (Ellis & Solander) Lamoroux, including the ancestrula and several ovicellate zooids; northern Ionian Sea, Gulf of Taranto, Porto Cesareo MPA; sample PCE10; 40°15'54"N, 17°52'38"E; 5–15 m; 2008; A. Sinagra leg.; scuba diving; C Biocoenosis; Paratypes: Italy • 1 dead colony fragment consisting of about a dozen zooids, some fertile; sample PCI10; same details as the holotype; PMC. B29b1. 20.11.2020; 1 dead colony fragment consisting of 9 zooids, 3 of which fertile; Ionian Sea, SE Sicily, Plemmirio MPA, Mazzere submarine cave; sample MZ1 (sediment); 37°00'18"N, 15°18'36"E; 23 m; 14 Sep. 2009; V. Di Martino leg.; scuba diving; C and GSO Biocoenoses; PMC. B29b2. 20.11.2020.
Diagnosis
Colony encrusting, multiserial. Autozooid frontal shield densely pustulose and centrally pseudoporous. Orifice transversely D-shaped; hinge-line smooth with rectangular condyles at corners; five or six oral spines, two visible in ovicellate zooids. Ascopore field circular to elliptical; ascopore opening bean-shaped, with small tongue and radial spines. Avicularium single, located at half zooidal length, directed laterally or slightly disto-laterally; crossbar complete; rostrum lanceolate, channelled. Ovicell produced by the distal zooid, personate with collar enclosing the ascopore and forming a bridge between the orifice and the ascopore, producing two secondary openings.
Description
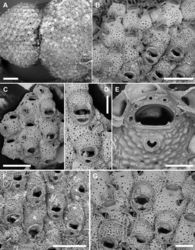
Colony encrusting, multiserial, unilaminar (Fig. 3A); interzooidal communications through four elliptical, lateral (two proximo- and two disto-lateral), and two rounded, distal pore chamber windows (38–67 × 16–21 µm).
Autozooids hexagonal, 460–522 (494±31, N = 3) × 411–476 (433±37, N = 3) µm (mean L/W = 1.16), boundaries marked by narrow, sinuous grooves and/or a raised rim. Frontal shield slightly convex, densely and evenly pustulose, with 11–25 circular (diameter 5–20 µm) pseudopores, irregularly distributed centrally; 3–6 marginal areolae, often indistinguishable from pseudopores (Fig. 3C, D).
Orifice transversely D-shaped, 83–95 (89±5, N = 6) × 141–170 (150±11, N = 6) µm (mean OL/OW = 0.60; mean ZL/OL = 5.47), outlined by a thin, raised (relative to the surrounding frontal shield) rim; hinge-line straight, smooth, with a pair of rectangular condyles at corners (Fig. 3E). Oral spines five or six (diameter of the base 18–27 μm), evenly spaced (Fig. 3C, E); proximalmost pair of spines sometimes visible in ovicellate autozooids, embedded between the proximal margin of the ooecium and the personate collar (Fig. 3D, G).
Ascopore field a very narrow, subcircular area of gymnocystal calcification, 35–42 × 46–70 μm, located 35–47 μm below the orifice, at the same level as the orifice but slightly raised relative to the adjacent frontal shield; opening bean-shaped, 32–37 × 9–19 μm, with a small, subcircular tongue projecting from distal edge and tiny radial denticles (Fig. 3E).
Avicularium single, relatively large, 134–190 (165±18, N = 10) × 86–109 (97±9, N = 10) μm (mean AvL/AvW = 1.70), located laterally, on either side, at about half zooidal length (Fig. 3B–D, G); crossbar complete, thin; rostrum long, lanceolate, channelled and open-ended, directed laterally or distolaterally, often raised distally on a smooth, gymnocystal cystid. Mandible lanceolate, 220–245 μm long, slightly longer than the rostrum (Fig. 3B, F).
Ovicell subglobular and slightly prominent, 147–239 (187±34, N = 8) × 262–343 (309±33, N = 8) μm (mean OvL/OvW = 0.60), produced by and continuous with frontal shield of distal zooid, personate, obscuring distal half of the orifice; calcification fabric similar to frontal shield but with smaller pseudopores (diameter 3–8 μm); distal boundary marked by a row of larger pseudopores; proximal margin of gymnocystal calcification forming a raised visor-like band (e.g., Fig. 3B–D). Personate structure of the ovicell with a collar enclosing the ascopore and forming a bridge of two fused flaps between the orifice and the ascopore, producing two secondary openings (Fig. 3D, F, G); secondary orifice transversely elliptical, 71–137 × 180–218 μm; secondary opening over the ascopore trumpet-like (38–52 × 83–145 μm).
Ancestrula tatiform partially overgrown (four spines still visible) and regenerated as an autozooid without avicularium.
Etymology
From the Latin prefix bi-, two/double, and the adjective collaris, pertaining to the neck, referring to the bridging structure between the orifice and the ascopore appearing as a double collar.
Remarks
Four species with personate ovicells are known to date from the Mediterranean. Microporella coronata (Audouin & Savigny, 1826) differs from the new species in having paired avicularia and a greater number of oral spines, always hidden in ovicellate zooids. Microporella browni Harmelin, Ostrovsky, Cáceres-Chamizo & Sanner, 2011, M. genisii (Audouin & Savigny, 1826), and M. orientalis Harmer, 1957 differ in having personate ovicell structures not enclosing the ascopore, and by the denticulation either on the distal or the proximal margin of the orifice.
Among all Microporella species known worldwide, the most similar to M. bicollaris sp. nov. is the eastern Pacific M. pontifica Osburn, 1952 reported from Clarion Island, Galapagos and the Gulf of California. Unfortunately, SEM images are not available for this species, but the original drawing (Osburn 1952[3]: pl. 44, fig. 5) shows the same personate structure of the ovicell observed in M. bicollaris sp. nov. However, the new species differs in having a larger avicularium placed more terminally relative to the lateral margin of the zooid, and by the presence of condyles in the orifice. The specimen drawn in Hayward and Ryland (1999[4]: fig. 136D) as Microporella ciliata “personate” form of Hincks (1880)[5], also appears similar to M. bicollaris sp. nov. However, the illustration in Hincks (1880)[5] appears different, but it is unclear whether Hayward and Ryland (1999)[4] examined any additional material. The north-eastern Atlantic specimens need to be revised to assess their conspecificity with the Mediterranean colonies.
Distribution and ecology
Microporella bicollaris sp. nov. is presently known only from Porto Cesareo MPA (Gulf of Taranto, southwestern Apulia, NE Ionian Sea), and the Mazzere submarine cave in the Plemmirio MPA (western Ionian Sea). All colonies are from shallow waters, collected in photophilic algae or found in a semi-dark submarine cave.
Original Description
- Martino, E; Rosso, A; 2021: Seek and ye shall find: new species and new records of Microporella (Bryozoa, Cheilostomatida) in the Mediterranean ZooKeys, 1053: 1-42. doi
Images
|
Other References
- ↑ Rosso A, Di Martino E, Sanfilippo R, Di Martino V (2013a) Bryozoan Communities and Thanatocoenoses from Submarine Caves in the Plemmirio Marine Protected Area (SE Sicily). In: Ernst A Schäfer P Scholz J (Eds) Bryozoan Studies 2010. Proceedings of the 15th IBA Conference, 2010 Kiel, Germany. Springer, Berlin, Heidelberg.Lecture Notes in Earth System Sciences143: 251–269. https://doi.org/10.1007/978-3-642-16411-8_17
- ↑ Rosso A, Sanfilippo R, Taddei Ruggiero E, Di Martino E (2013b) Faunas and ecological groups of Serpuloidea, Bryozoa and Brachiopoda from submarine caves in Sicily (Mediterranean Sea).Bollettino della Società Paleontologica Italiana52(3): 167–176.
- ↑ Osburn R (1952) Bryozoa of the Pacific coast of America, part 2, Cheilostomata-Ascophora.Report of the Allan Hancock Pacific Expeditions14: 271–611.
- ↑ 4.0 4.1 Hayward P, Ryland J (1999) Cheilostomatous Bryozoa. Part 2. Hippothoidea – Celleporoidea. Barnes RSK, Crothers JH (Eds) Synopses of the British Fauna (New Series) Field Studies Council, Shrewsbury 14: 1–416.
- ↑ 5.0 5.1 Hincks T (1880) A history of the British Marine Polyzoa.Van Voorst, London, 601 pp. [83 pls.] https://www.biodiversitylibrary.org/page/23373713