Tibicen neomexicensis
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Stucky2013ZooKeys337, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Stucky2013ZooKeys337">{{Citation See also the citation download page at the journal. |
Ordo: Hemiptera
Familia: Cicadidae
Genus: Tibicen
Name
Tibicen neomexicensis Stucky, 2013 sp. n. – Wikispecies link – ZooBank link – Pensoft Profile
Type locality
USA, New Mexico, Lincoln County, Lincoln National Forest, near the junction of Forest Road 105 and State Highway 37, 33.5287°N, 105.6939°W (datum: WGS84), elevation 2188 m, pinyon-juniper forest.
Holotype male. Pinned specimen (Figures 1–3). Original label: “NM: Lincoln Co. | Lincoln NF, FR 105 | 33.5287°N, 105.6939°W | May 31, 2012 7178 feet | Brian and Erin Stucky”. UCMC, specimen identifier UCMC 0046172. Paratypes. 8 males and 3 females, same label data as holotype; 2 males and 2 females, same label data as holotype except collected on May 30, 2012. The paratypes are currently housed in the UCMC and the author’s collection. Upon publication, paratypes will also be transferred to the ANSP, the Smithsonian National Museum of Natural History (NMNH), NMSU, and the SEMC.
Description
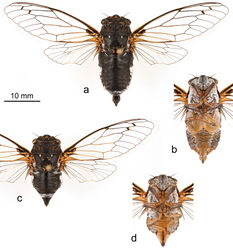
Head. Slightly wider than anterior margin of pronotum. Vertex and frons black, marked with orange-brown on the posterior margin near the eyes and immediately lateral of the lateral ocelli. Supra-antennal plates black dorsally with an orange-brown mark adjacent to the postclypeus, orange-brown ventrally marked with black immediately above the antennae, and orange-brown along the anterior margin except for immediately adjacent to the postclypeus. Antennae mostly black with distal margin of scape yellowish, proximal half of pedicel dark brown in some specimens. Dorsal surface of head sparsely covered with short golden hairs and with longer, silvery-white hairs behind the eyes. Ventral surface mostly covered with dense, silvery-white hairs. Postclypeus black, marked with orange-brown on the anterior-medial margin and with a triangular orange-brown mark adjacent to the frontoclypeal suture. Transverse grooves of postclypeus lined with pruinosity and silvery-white hairs. Anteclypeus black, yellowish posterolaterally, with a medial brown spot at the junction with the postclypeus. Lora mostly black, marked with yellow along the lateral margins. Genae black anteriorly, yellowish posteriorly where they border the lora. Proximal two thirds of rostrum yellowish, labrum and distal one third of rostrum black, with the apex extending posteriorly to the hind coxae.
Thorax. Pronotum black, marked faintly with dark brown between the paramedian and lateral fissures and between the lateral fissures and pronotal collar, brown markings often more extensive in females. Pronotal collar black, lined with orange along the anterior margin between the eyes and along the lateral margins, extending to the posterior margin and fading to black medially. Some specimens have the entire posterior margin lined with orange. Pronotum sparsely covered with fine golden hairs. Mesonotum black marked with orange as follows: two J-shaped lines following the parapsidal suture, a small spot at the terminal end of each anterior arm of the cruciform elevation, two C-shaped marks starting at the origin of the anterior arms of the cruciform elevation and curving medially then laterally towards the posterior arms, and a large mark near the base of each fore wing. Mesonotum with two small pruinose spots on the anterior margin just lateral of the parapsidal sutures, lateral margin also pruinose. Mesonotum sparsely covered with fine golden hairs, with longer silvery-white hairs in the depressions of the cruciform elevation and along the posterolateral margins. Visible portion of metanotum black, covered with silvery-white hairs laterally. Ventral surface of thorax often heavily pruinose and covered with silvery-white hairs, yellowish except for katepisternum 2, anterior portion of basisternum 2, anepimeron 2, central part of katepimeron 2, meron 2, anterior portions of trochantins 2 and 3, episternum 3, and basisternum 3, all of which are black.
Legs. Fore coxae orange marked with brown apically and with the anterolateral surface dark brown except along the margins. Middle and hind coxae orange marked with dark brown laterally. Coxae covered with silvery-white hairs and often pruinose. Trochanters orange, variably marked with brown. Femora orange, apex mostly yellow, brown ventrally, with longitudinal brown stripes that often merge apically and basally. Silvery-white hairs on femora mostly confined to brown markings. Femoral spines brown basally with dark brown apices. Tibiae orange ventrally, brown dorsally with brown markings expanded at the base, covered with silvery-white hairs. Tibial spurs and comb dark brown. Tarsi variable in color but usually dark brown dorsally and light brown to orange ventrally. Claws brown basally with dark brown apices.
Wings. Fore wings hyaline with 8 apical cells, crossveins r and r-m usually strongly infuscated. Costal margin yellow, C vein black, R+Sc vein black with posterior margin pale along the radial cell. Sc vein black beyond the node, subcostal margin brown to dark yellow. Basal cell mostly black, anterior and posterior borders yellow. M vein yellowish-black from its base to the junction with M1+2, black beyond. M3+4 yellowish-black. M1+2 yellowish-black becoming black apically. CuA vein yellow from its base to the junction with CuA2, yellowish-black beyond. CuA2 yellowish-black. CuP+1A and 2A+3A veins mostly yellow, ambient vein dark yellowish-black, remaining venation black. Hind wings hyaline with 6 apical cells. Sc+RA, RA, CuA between base and CuA2, and CuA2 veins mostly yellow to yellowish-orange. CuA between CuA2 and m-cu, and CuA1 veins yellow to yellowish-black. Ambient vein black marked with yellow along 1st cubital cell and 6th apical cell. Remaining venation mostly black or dark brown. 3rd anal cell gray marked with reddish-orange basally.
Opercula. Male opercula yellowish marked with black on the anterolateral and anteromedial margins, overlapping medially. Posterior margins smoothly rounded, not quite reaching the posterior margin of sternite II. Female opercula yellowish, becoming black anterolaterally. Posterior margin sinuate, reaching the anterior margin of sternite II. Meracanthus black basally with a yellow apex.
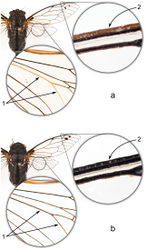
Abdomen. Dorsal surface of abdomen almost entirely black, sparsely covered with short golden and silvery hairs. Tergite 8 orange-brown laterally. Tergites 3-7 often marked with orange-brown laterally, markings usually strongest on tergite 3. Timbal covers black, sometimes dark brown centrally, completely concealing timbal. Timbal with 3 long ribs, 4 intercalary ribs, and an incomplete 4th long rib. Dorsal abdomen pruinose at the following locations: along the anteromedial margins of the timbal covers in males; along the anterolateral margins of tergite 2 in females; the lateral margins of tergites 3-7, most prominently on tergite 3; the lateral margins of tergite 8, often extending medially to cover most of the tergite. Sternites orange to yellowish, usually dark brown laterally and anterolaterally. Epipleurites orange to yellowish, indistinctly marked with dark brown or black. Ventroposterior portion of male sternite VIII dark brown.
Male terminalia. Pygofer black, becoming brown or yellowish laterally along the lobes, and with a small brown spot dorsally at the base of the dorsal beak. Dorsal beak not quite as long as anal styles. Anal styles black. Median lobe of uncus slender, black, strongly bent ventrally and terminating in a rounded point. Aedeagus reddish-brown.
Female terminalia. Abdominal segment 9 yellowish-orange ventrally, black dorsally starting at about the lateral mid-line. Dorsal beak about as long as anal styles. Sternite VII yellowish-orange, usually brown laterally, deeply notched at the middle of the posterior margin. Visible portion of gonocoxite IX yellowish-orange, indistinctly marked with brown near the posterior end. Ovipositor sheath black, ventromedial margins partially lined with orange. Ovipositor sheath extends posteriorly about as far as anal styles.
Measurements. All measurements are reported in mm as mean (range, standard deviation). Males (n = 11): head width: 8.3 (8.1–8.5, 0.13); pronotum width: 8.9 (8.5–9.3, 0.25); fore wing length: 28.1 (26.8–29.6, 0.76); fore wing width: 9.8 (9.3–10.5, 0.41); body length: 24.9 (23.1–26.3, 1.05). Females (n = 5): head width: 7.8 (7.7–7.9, 0.07); pronotum width: 8.4 (8.2–8.5, 0.14); fore wing length: 26.7 (26.2–27.4, 0.48); fore wing width: 9.1 (8.9–9.3, 0.20); body length: 20.0 (19.3–20.9, 0.64).
Etymology
The specific epithet refers to the U.S. state of New Mexico. As far as is currently known, Tibicen neomexicensis is endemic to this state. Morphometric comparison of Tibicen neomexicensis and Tibicen chiricahua Five morphometric measurements were taken for both Tibicen chiricahua and Tibicen neomexicensis: fore wing length, fore wing width, head width, pronotum width, and total body length. The correlation coefficient matrix for these five variables revealed that all five measurements were very strongly correlated with one another. All pairwise correlation coefficients excluding body length were > 0.91, and all pairwise correlation coefficients including body length were > 0.80. Body length in adult cicadas is not constant and instead varies according to a cicada’s abdominal posture, so the lower correlation coefficients for body length were not surprising. Given the high correlation among the five variables, analyzing each separately would have been largely redundant, so comparative analysis focused on fore wing length (Table 1). Fore wing length is invariant in adult cicadas and easily measured for either live or preserved specimens.
| variable | species | mean | 95% CI | range | std. dev. | n |
|---|---|---|---|---|---|---|
| fore wing length (mm) | Tibicen chiricahua, M | 31.3 | 30.6–32.0 | 29.4–33.1 | 1.06 | 11 |
| Tibicen neomexicanus, M | 28.1 | 27.6–28.6 | 26.8–29.6 | 0.76 | 11 | |
| Tibicen chiricahua, F | 29.1 | 28.0–30.2 | 28.0–30.1 | 0.88 | 5 | |
| Tibicen neomexicanus, F | 26.7 | 26.1–27.3 | 26.2–27.4 | 0.48 | 5 | |
| peak frequency (kHz) | Tibicen chiricahua | 7.12 | 6.56–7.67 | 5.73–8.47 | 0.82 | 11 |
| Tibicen neomexicanus | 7.27 | 7.02–7.52 | 6.07–7.81 | 0.45 | 15 | |
| amp. burst rate (bursts/s) | Tibicen chiricahua | 54 | 52.4–55.6 | 50.5–59.3 | 2.32 | 11 |
| Tibicen neomexicanus | 27.8 | 27.4–28.3 | 26.5–29.5 | 0.86 | 15 | |
| pulses per amplitude burst | Tibicen chiricahua | 5.02 | 4.54–5.51 | 3.92–6.42 | 0.723 | 11 |
| Tibicen neomexicanus | 8.34 | 7.70–8.98 | 7.25–11.33 | 1.066 | 13 | |
| phrase 1 length (s) | Tibicen chiricahua | 1.72 | 1.21–2.23 | 0.49–2.80 | 0.758 | 11 |
| Tibicen neomexicanus | 2.04 | 1.66–2.42 | 1.25–3.24 | 0.6 | 12 | |
| phrase 2 length (s) | Tibicen chiricahua | 7.82 | 6.95–8.68 | 6.02–10.83 | 1.286 | 11 |
| Tibicen neomexicanus | 6.68 | 5.77–7.59 | 4.86–10.96 | 1.575 | 14 | |
| phrase 3 length (s) | Tibicen chiricahua | 3.75 | 3.10–4.39 | 2.37–5.82 | 0.963 | 11 |
| Tibicen neomexicanus | 1.65 | 1.36–1.94 | 0.75–2.33 | 0.479 | 13 |
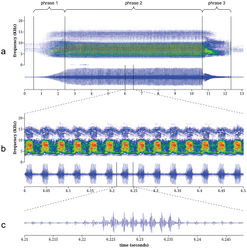
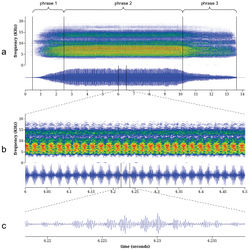
Description and comparison of the calling songs of Tibicen neomexicensis and Tibicen chiricahua Calling song of Tibicen neomexicensis. The calling song of Tibicen neomexicensis can be divided into three phrases, each of which consists of a continuous train of pulses (Figure 4). The first phrase represents the initial increase in amplitude as the cicada begins calling and lasts an average of 2.04 seconds (95% CI: 1.66–2.42; full descriptive statistics for all acoustic parameters are given in Table 1). The second phrase is the main phrase of the call and is produced at or near maximum amplitude. This phrase lasts an average of 6.68 seconds (95% CI: 5.77–7.59) and has a mean peak frequency of 7.27 kHz (95% CI: 7.02–7.52). The first two phrases are characterized by distinctive amplitude and frequency modulations that group pulses into regular “bursts” of high amplitude. During the main phrase, these amplitude bursts are delivered at a mean rate of 27.8 bursts/s (95% CI: 27.4–28.3) and each amplitude burst consists of 8.34 pulses on average (95% CI: 7.70–8.98). The amplitude and frequency modulations are accompanied by rapid dorso-ventral movements of the cicada’s abdomen. These movements modulate frequency and amplitude by changing the acoustic properties of the sound-producing system (Pringle 1954[1]). The third and final phrase of the call lasts an average of 1.65 seconds (95% CI: 1.36–1.94) and begins with a rapid initial drop in overall amplitude followed by a gradual decrease in amplitude until the calling song ends. During this final phrase, the amplitude and frequency modulations disappear, although the modulations sometimes briefly return as the call terminates. Calling song ofTibicen chiricahua. The calling song of Tibicen chiricahua is also naturally divided into three phrases (Figure 5). The first phrase is the initial crescendo as the call begins and lasts an average of 1.72 seconds (95% CI: 1.21–2.23). The second, main phrase of the call has a mean duration of 7.82 seconds (95% CI: 6.95–8.68) with a peak frequency of 7.12 kHz (95% CI: 6.56–7.67). The third phrase is a gradual decrescendo as the calling song terminates and lasts an average of 3.75 seconds (95% CI: 3.10–4.39). The entire call consists of an amplitude-modulated train of pulses. Pulses are grouped into high-amplitude bursts that, during the main phrase of the call, contain an average of 5.02 pulses per burst (95% CI: 4.54–5.51) and are delivered at a mean rate of 54.0 bursts/s (95% CI: 52.4–55.6). Comparison of calling songs. Comparison of acoustic parameters, song structure, and physical behavior during call production verified that the calls of these two species are distinct. First, the underlying structures of the amplitude modulations of the calls differ. The mean amplitude burst rate of the call of Tibicen chiricahua is nearly twice that of Tibicen neomexicensis (54.0 and 27.8 bursts/s, respectively, t = 37.4, p < 0.000001), and the amplitude bursts of Tibicen chiricahua contain about 3.3 fewer pulses per burst, on average, than those of Tibicen neomexicensis (5.02 and 8.34 pulses/burst, respectively, t = 18.0, p < 0.000001). There was no overlap in the ranges of observed values for either of these variables. Second, the phrasal structures of the calls also differ. The phrases in the call of Tibicen chiricahua are defined merely by the overall pattern of amplitude changes in the call and have a relatively uniform sound quality throughout, while the third phrase of the call of Tibicen neomexicensis is markedly different in quality from the other two phrases, lacking the characteristic modulations of phrases one and two. Furthermore, the beginning of the third phrase in Tibicen neomexicensis is usually marked by an abrupt drop in amplitude, but the amplitude decreases gradually and smoothly from the second to the third phrases of Tibicen chiricahua. Finally, the amplitude and frequency modulations in the call of Tibicen neomexicensis are a result of rapid dorso-ventral movements of the abdomen during the calling song, but no such movements were apparent in the calling behavior of Tibicen chiricahua.
The observed mean peak frequency of the main phrase of the call of Tibicen neomexicensis was slightly higher than that of Tibicen chiricahua, although the difference was not significant (7.27 and 7.12 kHz, respectively, t = 0.623, p = 0.539). Peak calling song frequency is constrained by body size for most cicadas, with larger cicadas having lower-frequency calls (Bennet-Clark and Young 1994[2]). Thus, given that Tibicen neomexicensis is smaller than Tibicen chiricahua but the two cicadas are not grossly dissimilar in size, it is not surprising that their peak call frequencies are similar, and even though the difference was not significant, the observed higher pitch of the call of Tibicen neomexicensis is consistent with the morphometric analysis.
Geographic distribution Tibicen chiricahua is more widely distributed than Tibicen neomexicensis, ranging from central and southeastern Arizona to southwestern New Mexico (Figure 6). Although not depicted in Figure 6, Tibicen chiricahua is also known from Chihuahua, Mexico (Sanborn 2007[3]). Tibicen neomexicensis is so far known only from the Sacramento Mountains in south-central New Mexico. All known localities for Tibicen chiricahua are west of the Rio Grande, while Tibicen neomexicensis has only been found east of the Rio Grande. Four museum specimens, representing two unique collecting localities, could not be conclusively identified. One, a female collected June 15, 1937 in “Big Bend Park,” Brewster Co, TX (TAMU), appeared to be Tibicen neomexicensis. However, Phillips and Sanborn (2007)[4] did not report any cicadas resembling Tibicen chiricahua in their intensive surveys of Big Bend National Park, so this record is doubtful. The other three specimens were two males and one female collected June, 1966 “near” Ciudad Cuauhtémoc, Chihuahua, Mexico (UCMC). These specimens are similar to Tibicen neomexicensis and Tibicen chiricahua, but differ in that all three have abdomens strongly marked with orange dorsolaterally. More information is needed regarding cicadas from this locality to properly determine their taxonomic status.
Original Description
- Stucky, B; 2013: Morphology, bioacoustics, and ecology of Tibicen neomexicensis sp. n., a new species of cicada from the Sacramento Mountains in New Mexico, U.S.A. (Hemiptera, Cicadidae, Tibicen) ZooKeys, 337: 49-71. doi
Images
|
Other References
- ↑ Pringle J (1954) A physiological analysis of cicada song. Journal of Experimental Biology 31: 525-560.
- ↑ Bennet-Clark H, Young D (1994) The scaling of song frequency in cicadas. Journal of Experimental Biology 191: 291-294.
- ↑ 3.0 3.1 Sanborn A (2007) New species, new records and checklist of cicadas from Mexico (Hemiptera: Cicadomorpha: Cicadidae). Zootaxa 1651: 1-42.
- ↑ Phillips P, Sanborn A (2007) Phytogeography influences biogeography of the Cicadidae. Acta Zoologica Sinica 53: 454-462.
![Figure 6. Geographic distribution of Tibicen chiricahua (orange circles) and Tibicen neomexicensis sp. n. (yellow triangles), estimated from field observations and museum specimens. Green regions indicate pinyon-juniper habitats. The gray region represents the Albuquerque Basin and Chihuahuan Desert. Tibicen chiricahua is also found in Mexico (Sanborn 2007[3]).](https://species-id.net/o/thumb.php?f=ZooKeys-337-049-g006.jpg&width=250)