Niumbaha
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Reeder2013ZooKeys285, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Reeder2013ZooKeys285">{{Citation See also the citation download page at the journal. |
Ordo: Chiroptera
Familia: Vespertilionidae
Name
Niumbaha Reeder et al. gen. n. – Wikispecies link – ZooBank link – Pensoft Profile
Etymology
The name is the Zande word for ‘rare/unusual’. This name was chosen because of the rarity of capture for this genus, despite its wide distribution throughout West and Central Africa, and for the unusual and striking appearance of this bat. Zande is the language of the Azande people, who are the primary ethnic group in Western Equatoria State in South Sudan (where our recent specimen was collected). The homeland of the Azande extends westwards into Democratic Republic of the Congo, where superba has also been collected (the holotype and another recent capture), and into southeastern Central African Republic. Gender: feminine.
Type species
Glauconycteris superba Hayman, 1939; by monotypy.
Diagnosis
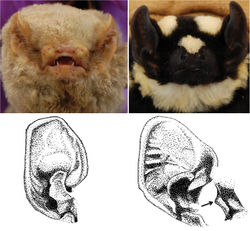
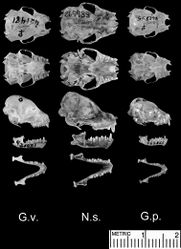
Among vespertilionids, Niumbaha bears closest comparison with species of Glauconycteris (the type species of which is Glauconycteris poensis), to which it is apparently closely related, but it has a considerably larger skull and is more strikingly patterned compared to any member of Glauconycteris (its patterning most closely approaching the Asian vespertilionid genus Scotomanes). It lacks various of the most exaggeratedly derived traits (specializations) that uniquely unite the species of Glauconycteris among African vespertilionids, including the excessively foreshortened rostrum, moderately to highly reduced relative canine size, and very elongate wing tips (second wing phalanxes) of Glauconycteris (Rosevear 1965[1]). Externally, Niumbaha is immediately distinguished from all other African vespertilionid bats by its distinct coloration pattern, including pale yellow spots and stripes on an otherwise dark black pelage (Fig. 2, Fig. 3, and detailed descriptions below). While Hayman (1939[2]:222) noted that, “in general form Glauconycteris superba does not differ from other Glauconycteris,” we find that most external features are in fact different from Glauconycteris sensu stricto. The ears of Niumbaha are more robust and subquadrangular, contain a larger free lobe at the inner margin, and contain a more strongly curved tragus than Glauconycteris (Fig. 3). The muzzle of Niumbaha is more robust than Glauconycteris sensu stricto and contains nostrils that open more to the front than to the side (Fig. 3). The wingtips in Niumbaha are longer than in most other African vespertilionids in that phalanx 2 of the third digit is longer than phalanx 1, yet remain considerably shorter than in the characteristically long-wingtipped Glauconycteris (ratio of Ph2/Ph1 in Niumbaha, at 1.15 ± 0.05 SD, is significantly shorter than Glauconycteris, at 1.51 ± 0.12 SD; Fig. 4). Niumbaha shares its dental formula with Glauconycteris, at 2.1.1.3/3.1.2.3 = 32, but is overall significantly larger than species of Glauconycteris in all characters, with a total skull length of greater than 16.0 mm (Table 2; Fig. 5). While the rostrum of Glauconycteris is short and generally rises in an even plane from the incisors to the occiput, the frontal region of the skull in Niumbaha is excavated or ‘hollowed out’, with the upper surface of the longer rostrum largely flat and roughly parallel to the upper toothrows (see Fig. 5). Additionally, the skull is relatively less broad and less domed and more elongate than in Glauconycteris (indicated by ratios of the mastoid width, breadth of the braincase, height of the braincase, and zygomatic breadth to the greatest length of the skull (Table 2)), although the anterior portion of the rostrum is relatively broader (indicated by the ratio of the width at the upper canines to the width at the last molar (M3-M3)).
| Character | Niumbaha superba* | Glauconycteris alboguttata | Glauconycteris argentata | Glauconycteris beatrix | Glauconycteris curryae | Glauconycteris humeralis | Glauconycteris poensis | Glauconycteris cf. poensis | Glauconycteris variegata | Scotophilus leucogaster | Scotophilus viridis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ML-III | |||||||||||
| X‾ ± SD | 44.7 ± 2.3 | 39.8 | 41.7 ± 0.7 | 37.8 ± 1.9 | 35.0 | - | 38.4 ± 1.3 | 42.3 ± 1.1 | 42.3 ± 1.3 | 50.8 ± 1.4 | 46.8 ± 2.5 |
| Min-max | 42.0–47.4 | - | 40.8–42.3 | 35.2–39.6 | - | - | 36.4–39.6 | 40.8–44.3 | 40.5–44.1 | 48.6–52.4 | 41.5–50.1 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| DIII-1PL | |||||||||||
| X‾ ± SD | 20.4 ± 1.3 | 16.0 | 15.6 ± 0.6 | 13.4 ± 0.9 | 13.6 | - | 13.9 ± 0.9 | 15.6 ± 0.7 | 16.6 ± 0.6 | 18.6 ± 0.5 | 17.1 ± 1.1 |
| Min-max | 18.7–22.0 | - | 15.0–16.3 | 12.3–14.5 | - | - | 12.9–15.2 | 14.9–16.7 | 15.7–17.4 | 18.1–19.6 | 15.7–19.2 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| DIII-2PL | |||||||||||
| X‾ ± SD | 23.4 ± 1.1 | 22.0 | 25.7 ± 0.6 | 21.3 ± 1.6 | 19.5 | - | 21.3 ± 1.0 | 24.1 ± 0.8 | 22.8 ± 1.4 | 14.6 ± 0.8 | 13.9 ± 1.2 |
| Min-max | 22.4–24.3 | - | 24.8–26.2 | 19.7–22.9 | - | - | 20.1–22.9 | 22.5–25.6 | 20.7–24.5 | 13.4–15.5 | 11.9–15.7 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| ML-IV | |||||||||||
| X‾ ± SD | 43.4 ± 2.5 | 35.8 | 39.1 ± 1.1 | 34.1 ± 1.8 | 32.5 | - | 34.9 ± 1.1 | 39.3 ± 1.4 | 40.8 ± 1.3 | 48.9 ± 1.6 | 45.6 ± 2.3 |
| Min-max | 40.6–46.4 | - | 37.7–40.3 | 31.6–36.0 | - | - | 33.6–36.4 | 37.0–41.8 | 39.4–42.5 | 46.4–50.6 | 41.4–48.9 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| DIV-1PL | |||||||||||
| X‾ ± SD | 13.5 ± 1.2 | 11.8 | 11.7 ± 0.4 | 10.1 ± 0.7 | 8.8 | - | 11.1 ± 0.4 | 11.6 ± 0.7 | 12.7 ± 0.9 | 13.7 ± 1.1 | 13.5 ± 1.0 |
| Min-max | 12.2–15.0 | - | 11.4–12.1 | 9.1–10.8 | - | - | 10.5–11.4 | 10.8–12.2 | 11.2–13.9 | 11.3–15.1 | 12.1–14.8 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| DIV-2PL | |||||||||||
| X‾ ± SD | 10.1 ± 0.9 | 10.8 | 11.8 ± 0.6 | 11.5 ± 1.1 | 12.0 | - | 10.8 ± 0.9 | 11.6 ± 0.8 | 12.3 ± 0.8 | 10.2 ± 0.3 | 9.1 ± 0.9 |
| Min-max | 9.0–10.8 | - | 11.2–12.4 | 10.1–12.6 | - | - | 9.9–11.9 | 10.6–12.5 | 10.9–13.5 | 9.8–10.7 | 7.2–10.2 |
| n | 4 | 1 | 4 | 4 | 1 | - | 4 | 5 | 8 | 8 | 9 |
| ML-V | |||||||||||
| X‾ ± SD | 38.8 ± 2.8 | 31.2 | 35.5 ± 0.9 | 32.5 ± 1.2 | 29.9 | - | 32.1 ± 0.8 | 35.5 ± 0.8 | 38.6 ± 1.5 | 47.1 ± 1.6 | 42.9 ± 1.9 |
| Min-max | 35.5–42.0 | - | 34.3–36.3 | 31.3–34.2 | - | - | 30.6–32.8 | 33.7–37.0 | 36.1–40.4 | 43.9–49.4 | 39.5–45.8 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| DV-1PL | |||||||||||
| X‾ ± SD | 8.8 ± 1.2 | 9.6 | 9.6 ± 0.4 | 9.4 ± 0.4 | 8.4 | - | 9.8 ± 0.5 | 10.3 ± 1.0 | 10.6 ± 0.6 | 10.2 ± 0.9 | 9.0 ± 0.6 |
| Min-max | 7.6–10.4 | - | 9.2–10.2 | 8.8–9.7 | - | - | 9.1–10.3 | 9.1–11.4 | 9.7–11.4 | 8.9–11.6 | 7.9–9.6 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| DV-2PL | |||||||||||
| X‾ ± SD | 7.5 ± 0.7 | 7.8 | 8.7 ± 0.6 | 7.4 ± 0.5 | 7.6 | - | 8.1 ± 0.5 | 7.8 ± 0.4 | 8.3 ± 0.9 | 6.4 ± 0.3 | 6.4 ± 0.7 |
| Min-max | 6.8–8.2 | - | 8.3–9.5 | 6.8–7.9 | - | - | 7.5–8.8 | 6.7–8.4 | 7.2–9.8 | 5.9–6.9 | 5.7- 7.4 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| DIII-2PL/1PL | |||||||||||
| X‾ ± SD | 1.1 ± 0.1 | 1.4 | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.4 | - | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0 | 0.8 ± 0.1 | 0.8 ± 0.01 |
| Min-max | 1.1–1.2 | - | 1.5–1.7 | 1.5–1.7 | - | - | 1.4–1.7 | 1.5–1.6 | 1.3–1.4 | 0.7–0.9 | 0.7–0.9 |
| n | 4 | 1 | 4 | 4 | 1 | - | 5 | 5 | 8 | 8 | 9 |
| DIV-2PL/1PL | |||||||||||
| X‾ ± SD | 0.8 ± 0.1 | 0.9 | 1.0 ± 0.0 | 1.1 ± 0.1 | 1.4 | - | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| Min-max | 0.7–0.8 | - | 1.0–1.1 | 1.0–1.3 | - | - | 0.9–1.1 | 0.9–1.1 | 0.9–1.1 | 0.7–0.8 | 0.6–0.8 |
| n | 4 | 1 | 4 | 4 | 1 | - | 4 | 5 | 8 | 8 | 9 |
| GLS | |||||||||||
| X‾ ± SD | 16.8 ± 0.6** | 13.3 | 12.7 ± 0.3 | 11.4 ± 0.2 | 12.2 | 11.1 ± 0 | 12.3 ± 0.3 | - | 13.9 ± 0.3 | 20.5 ± 0.3 | 18.0 ± 0.7 |
| Min-max | 16.2–17.4 | 13.2–13.4 | 12.0–13.3 | 11.2–11.6 | - | 11.1–11.1 | 12.0–12.7 | - | 13.4–14.4 | 20.1–20.9 | 17.0–18.4 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 6 | - | 23 | 7 | 4 |
| CIL | |||||||||||
| X‾ ± SD | 15.6 ± 0.4** | 12.8 | 12.3 ± 0.3 | 11.1 ± 0.3 | 11.1 | 10.9 ± 0.1 | 11.9 ± 0.3 | - | 13.3 ± 0.3 | 18.0 ± 0.2 | 16.3 ± 0.5 |
| Min-max | 15.4–16.2 | 12.7–12.9 | 11.7–12.5 | 10.9–11.5 | - | 10.8–11.0 | 11.5–12.4 | - | 12.8–13.8 | 17.7–18.3 | 15.6–16.6 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 6 | - | 23 | 7 | 4 |
| CCL | |||||||||||
| X‾ ± SD | 16.0 | 12.4 | 11.9 ± 0.4 | 10.9 ± 0.3 | 10.7 | 10.8 ± 0.3 | 11.5 ± 0.3 | - | 12.9 ± 0.3 | 17.5 ± 0.3 | 15.8 ± 0.4 |
| Min-max | - | 12.4–12.4 | 11.0–12.2 | 10.7–11.2 | - | 10.5–11.0 | 11.1–12.0 | - | 12.3–13.4 | 17.1–17.9 | 15.3–16.3 |
| n | 1 | 2 | 13 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| Palatal length | |||||||||||
| X‾ ± SD | 5.9 ± 0.4** | 5.3 | 4.8 ± 0.2 | 4.4 ± 0.1 | - | 4.6 ± 0.2 | 4.8 ± 0.5 | - | 5.2 ± 0.3 | 7.1 ± 0.1 | 6.5 ± 0.5 |
| Min-max | 5.5–6.5 | 5.1–5.5 | 4.4–5.3 | 4.3–4.5 | - | 4.4–4.8 | 4.4–5.5 | - | 4.8– 6.0 | 6.9–7.3 | 6.1–7.2 |
| n | 4 | 2 | 13 | 3 | - | 3 | 4 | - | 22 | 7 | 4 |
| ZB | |||||||||||
| X‾ ± SD | 11.4 ± 0.5** | 9.5 | 9.0 ± 0.2 | 8.3 ± 0.2 | 8.5 | 8.2 | 8.6 ± 0.2 | - | 10.2 ± 0.3 | 13.1 ± 0.4 | 12.0 ± 0.4 |
| Min-max | 11.0–11.9 | 9.4–9.5 | 8.6–9.2 | 8.1–8.4 | - | 8.0–8.3 | 8.4–8.9 | - | 9.5–10.9 | 12.7–13.8 | 11.5–12.3 |
| n | 4 | 2 | 10 | 3 | 1 | 2 | 7 | - | 23 | 7 | 4 |
| Mastoid width | |||||||||||
| X‾ ± SD | 9.6 ± 0.2** | 8.4 | 8.2 ± 0.3 | 7.5 ± 0.1 | 7.3 | 7.3 ± 0.2 | 7.7 ± 0.2 | - | 8.9 ± 0.2 | 11.5 ± 1.0 | 10.2 ± 0.4 |
| Min-max | 9.5–9.9 | 8.4 – 8.4 | 7.9–8.5 | 7.4–7.6 | - | 7.1–7.4 | 7.5–8.0 | - | 8.4–9.4 | 9.3–12.3 | 9.6–10.5 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 7 | - | 23 | 7 | 4 |
| BBC | |||||||||||
| X‾ ± SD | 8.7 ± 0.3** | 7.8 | 7.6 ± 0.2 | 6.9 ± 0.1 | 6.8 | 6.8 ± 0.1 | 7.2 ± 0.3 | - | 8.0 ± 0.2 | 9.2 ± 0.2 | 8.3 ± 0.2 |
| Min-max | 8.5–9.0 | 7.7–7.9 | 7.4–8.0 | 6.9–7.0 | - | 6.7–6.9 | 6.8–7.4 | - | 7.6–8.4 | 8.8–9.4 | 8.1–8.5 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| HBC | |||||||||||
| X‾ ± SD | 6.9 ± 0.3** | 5.8 | 5.7 ± 0.2 | 5.1 ± 0.1 | 4.9 | 5.1 ± 0.1 | 5.4 ± 0.2 | - | 6.0 ± 0.1 | 8.2 ± 0.3 | 6.8 ± 0.5 |
| Min-max | 6.6–7.3 | 5.8 – 5.8 | 5.5–6.0 | 4.9–5.2 | - | 5.0–5.2 | 5.1–5.6 | - | 5.7–6.2 | 7.7–8.6 | 6.1–7.1 |
| n | 4 | 2 | 11 | 3 | 1 | 3 | 7 | - | 23 | 7 | 4 |
| Interorbital width | |||||||||||
| X‾ ± SD | 6.4 ± 0.2** | 5.7 | 5.4 ± 0.1 | 4.6 ± 0.1 | 4.6 | 4.6 ± 0.2 | 5.3 ± 0.1 | - | 6.0 ± 0.3 | 8.1 ± 0.3 | 7.1 ± 0.4 |
| Min-max | 6.2–6.7 | 5.6–5.8 | 5.3–5.6 | 4.6–4.7 | - | 4.4–4.7 | 5.1–5.4 | - | 5.6–6.9 | 7.6–8.4 | 6.5–7.3 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 7 | - | 23 | 7 | 4 |
| POP | |||||||||||
| X‾ ± SD | 6.4 ± .3** | 5.8 | 5.5 ± 0.1 | 4.9 ± 0.1 | 4.7 | 4.6 ± 0.3 | 5.3 ± 0.1 | - | 6.0 ± 0.2 | 7.9 ± 0.2 | 6.9 ± 0.3 |
| Min-max | 6.1–6.9 | 5.8–5.8 | 5.3–5.8 | 4.8–5.0 | - | 4.4–4.9 | 5.1–5.5 | - | 5.7–6.4 | 7.6–8.2 | 6.5–7.3 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 7 | - | 23 | 7 | 4 |
| POC | |||||||||||
| X‾ ± SD | 4.8 ± 0.1** | 4.7 | 4.8 ± 0.4 | 4.3 ± 0.0 | 4.4 | 4.1 ± 0.1 | 4.2 ± 0.2 | - | 4.6 ± 0.1 | 5.0 ± 0.2 | 4.3 ± 0.2 |
| Min-max | 4.7–5.0 | 4.5–4.8 | 4.5–5.9 | 4.3–4.3 | - | 4.0–4.1 | 3.9–4.4 | - | 4.2–4.8 | 4.6–5.2 | 4.1–4.5 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| M3-M3 | |||||||||||
| X‾ ± SD | 8.0 ± 0.3** | 6.5 | 6.0 ± 0.2 | 5.4 ± 0.3 | 5.6 | 5.2 ± 0.1 | 5.8 ± 0.2 | - | 6.8 ± 0.2 | 8.5 ± 0.2 | 7.7 ± 0.1 |
| Min-max | 7.5–8.2 | 6.4–6.5 | 5.8–6.2 | 5.2–5.7 | - | 5.2–5.3 | 5.5–6.1 | - | 6.6–7.2 | 8.3–8.7 | 7.6–7.9 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 7 | - | 23 | 7 | 4 |
| C-M3 | |||||||||||
| X‾ ± SD | 6.0 ± 0.2** | 4.4 | 4.1 ± 0.1 | 3.9 ± 0.1 | 4.0 | 3.8 ± 0.1 | 4.1 ± 0.1 | - | 4.7 ± 0.1 | 6.6 ± 0.1 | 5.9 ± 0.2 |
| Min-max | 5.8–6.2 | 4.3–4.4 | 3.9–4.2 | 3.8–4.0 | - | 3.7–3.9 | 4.0–4.2 | - | 4.5–5.0 | 6.5–6.7 | 5.7–6.0 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| C-C | |||||||||||
| X‾ ± SD | 6.0 ± 0.2** | 4.8 | 4.3 ± 0.1 | 3.9 ± 0.1 | 3.5 | 3.7 ± 0.1 | 4.4 ± 0.2 | - | 4.8 ± 0.2 | 6.4 ± 0.2 | 5.7 ± 0.2 |
| Min-max | 5.8–6.2 | 4.8–4.9 | 4.1–4.5 | 3.9–4.0 | - | 3.6–3.8 | 4.0–4.6 | - | 4.4–5.2 | 6.2–6.6 | 5.4–5.9 |
| n | 4 | 2 | 12 | 3 | 1 | 3 | 7 | - | 23 | 7 | 4 |
| Mandible | |||||||||||
| X‾ ± SD | 12.3 ± 0.5** | 9.6 | 9.0 ± 0.2 | 8.3 ± 0.2 | 8.2 | 8.6 ± 0.5 | 8.7 ± 0.3 | - | 10.1 ± 0.2 | 14.1 ± 0.3 | 12.7 ± 0.2 |
| Min-max | 11.6–12.7 | 9.6–9.6 | 8.7–9.3 | 8.2–8.5 | - | 8.2–9.1 | 8.4–9.1 | - | 9.8–10.5 | 13.6–14.5 | 12.4–12.9 |
| n | 4 | 2 | 11 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| c-m3 | |||||||||||
| X‾ ± SD | 6.7 ± 0.2** | 5.0 | 4.6 ± 0.2 | 4.1 ± 0.3 | 4.4 | 4.3 ± 0.4 | 4.5 ± 0.2 | - | 5.3 ± 0.2 | 7.5 ± 0.2 | 6.6 ± 0.1 |
| Min-max | 6.4–6.9 | 4.9–5.1 | 4.3–4.9 | 3.9–4.5 | - | 4.0–4.8 | 4.2–4.7 | - | 5.1–5.6 | 7.2–7.7 | 6.5–6.8 |
| n | 4 | 2 | 11 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| Height of the upper canine | |||||||||||
| X‾ ± SD | 2.8 | 2.2 | 1.9 ± 0.1 | 1.3 ± 0.1 | 1.4 | 1.2 ± 0.1 | 1.8 ± 0.1 | - | 2.2 ± 0.2 | 3.5 ± 0.4 | 3.1 ± 0.2 |
| Min-max | - | 2.1–2.2 | 1.7–2.1 | 1.2–1.4 | - | 1.1–1.3 | 1.6–1.9 | - | 1.8–2.4 | 2.9–3.9 | 2.9–3.4 |
| n | 1 | 2 | 12 | 3 | 1 | 3 | 6 | - | 22 | 7 | 4 |
| Thickness of the upper canine | |||||||||||
| X‾ ± SD | 1.3 | 0.9 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.7 | 0.7 ± 0.1 | 0.8 ± 0 | - | 0.9 ± 0.1 | 1.6 ± 0.2 | 1.2 ± 0.1 |
| Min-max | - | 0.9–0.9 | 0.5–1.0 | 0.6–0.8 | - | 0.6–0.7 | 0.8–0.8 | - | 0.7–1.0 | 1.4–1.9 | 1.2–1.4 |
| n | 1 | 2 | 12 | 3 | 1 | 3 | 6 | - | 23 | 7 | 4 |
| WM3 | |||||||||||
| X‾ ± SD | 1.9 | 1.5 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.4 | 1.3 ± 0 | 1.4 ± 0.1 | - | 1.4 ± 0.1 | 2.2 ± 0.2 | 2.0 ± 0.1 |
| Min-max | - | 1.5–1.5 | 1.4–1.5 | 1.2–1.4 | - | 1.3–1.3 | 1.3–1.4 | - | 1.6–2.0 | 2.1–2.5 | 1.9–2.0 |
| n | 1 | 2 | 12 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| WM2 | |||||||||||
| X‾ ± SD | 2.4 | 1.7 | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.5 | 1.3 ± 0 | 1.5 ± 0.1 | - | 1.8 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 |
| Min-max | - | 1.6–1.8 | 1.4–1.5 | 1.2–1.3 | - | 1.3–1.3 | 1.4–1.6 | - | 1.6–2.0 | 2.1–2.4 | 2.1–2.4 |
| n | 1 | 2 | 12 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| MRL | |||||||||||
| X‾ ± SD | 2.9 | 2.1 | 2.1 ± 0.3 | 1.6 ± 0.3 | 2.3 | 1.8 ± 0.3 | - | - | 2.1 ± 0.2 | - | - |
| Min-max | - | 1.9–2.2 | 1.5–2.4 | 1.4–1.9 | - | 1.5–2.0 | - | - | 1.6–2.5 | - | - |
| n | 1 | 2 | 12 | 3 | 1 | 3 | - | - | 23 | - | - |
| I-M2 alv | |||||||||||
| X‾ ± SD | 6.8 | 4.9 | 4.6 ± 0.2 | 4.3 ± 0.3 | 4.3 | 4.3 ± 0.2 | 4.6 ± 0.1 | - | 5.2 ± 0.2 | 7.2 ± 0.2 | 6.4 ± 0.1 |
| Min-max | - | 4.9–4.9 | 4.2–4.8 | 4.1–4.6 | - | 4.1–4.4 | 4.4–4.8 | - | 4.7–5.4 | 6.9–7.5 | 6.3–6.6 |
| n | 1 | 2 | 12 | 3 | 1 | 3 | 7 | - | 24 | 7 | 4 |
| Mastoid width/GLS | |||||||||||
| X‾ ± SD | 0.57 ± 0.01** | 0.63 | 0.65 ± 0.01 | - | 0.60 | 0.65 ± 0.01 | 0.63 ± 0.01 | - | 0.64 ± 0.01 | 0.56 ± 0.04 | 0.57 ± 0.00 |
| Min-max | 0.56–0.59 | 0.63–0.64 | 0.63–0.67 | - | - | 0.64–0.67 | 0.62–0.64 | - | 0.61–0.66 | 0.46–0.60 | 0.56–0.57 |
| n | 4 | 2 | 12 | - | 1 | 3 | 6 | - | 22 | 7 | 4 |
| BBC/GLS | |||||||||||
| X‾ ± SD | 0.52 ± 0.00** | 0.59 | 0.60 ± 0.01 | - | 0.56 | 0.61 ± 0.01 | 0.58 ± 0.01 | - | 0.58 ± 0.01 | 0.45 ± 0.01 | 0.46 ± 0.02 |
| Min-max | 0.52–0.52 | 0.58–0.60 | 0.58–0.63 | - | - | 0.60–0.62 | 0.56–0.60 | - | 0.55–0.61 | 0.43–0.46 | 0.46–0.47 |
| n | 4 | 2 | 12 | - | 1 | 3 | 6 | - | 23 | 7 | 4 |
| HBC/GLS | |||||||||||
| X‾ ± SD | 0.41 ± 0.01** | 0.44 | 0.45 ± 0.12 | - | 0.40 | 0.46 ± 0.01 | 0.43 ± 0.01 | - | 0.43 ± 0.01 | 0.40 ± 0.01 | 0.39 ± 0.02 |
| Min-max | 0.41–0.42 | 0.43–0.44 | 0.43–0.47 | - | - | 0.45–0.46 | 0.42–0.45 | - | 0.41–0.45 | 0.38–0.41 | 0.36–0.39 |
| n | 4 | 2 | 11 | - | 1 | 3 | 6 | - | 22 | 7 | 4 |
| ZB/GLS | |||||||||||
| X‾ ± SD | 0.68 ± 0.01 | 0.71 | 0.71 ± 0.03 | - | 0.70 | 0.73 | 0.70 ± 0.02 | - | 0.73 ± 0.02 | 0.64 ± 0.02 | 0.67 ± 0.01 |
| Min-max | 0.67–0.69 | 0.71–0.71 | 0.68–0.77 | - | - | 0.72–0.75 | 0.67–0.73 | - | 0.69–0.77 | 0.62–0.66 | 0.66–0.68 |
| n | 4 | 2 | 10 | - | 1 | 2 | 6 | - | 22 | 7 | 4 |
| C-C/M3-M3 | |||||||||||
| X‾ ± SD | 0.76 ± 0.01** | 0.74 | 0.72 ± 0.02 | - | 0.63 | 0.71 ± 0.02 | 0.76 ± 0.04 | - | 0.70 ± 0.02 | 0.76 ± 0.03 | 0.73 ± 0.03 |
| Min-max | 0.74–0.77 | 0.73–0.76 | 0.69–0.76 | - | - | 0.69–0.73 | 0.69–0.82 | - | 0.65- 0.75 | 0.72–0.80 | 0.70–0.77 |
| n | 4 | 2 | 12 | - | 1 | 3 | 7 | - | 23 | 7 | 4 |
Material examined
The collection of a new specimen of Niumbaha superba in South Sudan (USNM 586592) in July 2012 allowed for the examination of a live bat and for the preservation of an intact specimen in fluid. This bat was captured in a single-high ground-level mist net next to a stagnant pool of water on a rocky grasslands plateau. This plateau, located at 04°52.643'N, 027°40.557'E (elevation ~ 720 m) is surrounded by secondary thicket forest and is within the boundaries of Bangangai Game Reserve, Ezo County, Western Equatoria State. Data for previously collected specimens of Niumbaha superba were taken from Hayman (1939[2], 1947) and from Randolph L. Peterson’s notes, provided by Judith Eger at the Royal Ontario Museum. An additional specimen was recently collected in the Democratic Republic of the Congo and reported by Gembu Tungaluna (2012)[3].
Data for Niumbaha superba were compared to those of various species of Glauconycteris, as summarized in Table 2. Additionally, for the wingtip analysis, comparisons with other, more ‘typical’ West African vespertilionids of similar size to Niumbaha superba (Scotophilus leucogaster and Scotophilus viridis) were made. Species/specimens examined: Glauconycteris alboguttata J. A. Allen, 1917 (2): Cameroon (AMNH 236329, USNM 598588); Glauconycteris argentata (Dobson, 1875) (14): Cameroon (AMNH 23624, AMNH 23625, AMNH 23627, AMNH 23628), Democratic Republic of the Congo (AMNH 120328, AMNH 120332, USNM 535398), Kenya (USNM 268759), Tanzania (AMNH 55545, AMNH 55546, AMNH 55548, USNM 297476, USNM 297477, USNM 297478); Glauconycteris beatrix Thomas, 1901 (4): Cameroon (USNM 511928, USNM 511929), Gabon (USNM 584723), Ghana (USNM 420078); Glauconycteris curryae Eger and Schlitter, 2001 (1): Gabon (USNM 584724); Glauconycteris humeralis J.A. Allen, 1917 (3): Democratic Republic of the Congo (AMNH 49014, AMNH 49312, AMNH 49315); Glauconycteris poensis (Gray, 1842) (12): Ivory Coast (USNM 429953, USNM 429954, USNM 429955, USNM 468192), Ghana (USNM 479528, USNM 479529, USNM 479530, USNM 479531, USNM 479533), Nigeria (AMNH 273244), Togo (USNM 437777, USNM 437778); Glauconycteris cf. poensis (6): South Sudan (new country record) (USNM 586596, USNM 586597, USNM 586598, USNM 586599, USNM 586600, USNM 586601), Glauconycteris variegata (Tomes, 1861) (27): Benin (USNM 421480, USNM 421481), Botswana (USNM 518696, USNM 518697), Democratic Republic of the Congo (AMNH 49060, AMNH 49061, AMNH 49062, AMNH 49063, AMNH 49066, AMNH 49067, AMNH 49068, AMNH 49070, AMNH 49195, AMNH 49313), Ghana (USNM 420077, USNM 424900), Kenya (AMNH 238490), Mozambique (USNM 304844), Nigeria (USNM 378863, USNM 378864, USNM 378865), South Africa (AMNH 257397), South Sudan (USNM 586593, USNM 586594, USNM 586595, USNM 590905), Uganda (AMNH 184228); Niumbaha superba (Hayman, 1939) (4): Democratic Republic of the Congo (RMCA 14.765), Ivory Coast (RMCA A9363), Ghana (BMNH 47.10), South Sudan (USNM 586592); Scotophilus leucogaster (Cretzschmar, 1830) (8): Benin (USNM 421421, USNM 421424, USNM 421425), Burkina Faso (USNM 450698, USNM 452887, USNM 452889, USNM 503955), Sierra Leone (USNM 547030); Scotophilus viridis (Peters, 1852) (9): Ivory Coast (USNM 468194, USNM 468195, USNM 468199), Mozambique (USNM 365411, USNM 365412, USNM 365413, USNM 365414, USNM 365417, USNM 365418). Museum abbreviations and information: USNM: National Museum of Natural History, Smithsonian Institution, (Washington, D.C., USA); AMNH: American Museum of Natural History (New York, USA); BMNH: British Museum of Natural History (London, UK); RMCA: Royal Museum for Central Africa (Tervuren, Belgium).
Notes
Species of Glauconycteris are quickly recognized by a variety of distinctive traits, many of which are shared with the monotypic Niumbaha. Below we examine each of these traits, highlighting similarities and differences between Niumbaha and Glauconycteris.
Coloration, pattern, and body size: Hayman (1939)[2] described and illustrated the coloration and patterning of this bat in detail, based upon the first specimen collected in Belgian Congo (now Democratic Republic of the Congo) (RMCA 14.765). He noted the presence of: (1) two sets of stripes on the dorsum - one set of “lanceolate stripes” found on each side of the median dorsal line of the back starting near the base of the neck and tapering to an end near the middle of the back, and one set of longer, narrower stripes on either side of the body, each commencing a little in advance of and lateral to the ends of medial stripes and each terminating just short of the root of the tail; (2) a set of stripes that begin on the dorsal side of each shoulder and run over the shoulder to the venter where they widen and run the lateral length of the venter joining and widening in the perineal region; (3) a wide throat band that connects to the shoulder/venter stripe, and (4) three spots – one roughly circular patch on the top of the muzzle between the eyes and one at each side of the face at the base of each ear.
In 1947, Hayman described the second specimen collected, this time from the Gold Coast (Ghana) (BMNH 47.10). Hayman found the markings of this specimen sufficiently different from the holotype of superba that he erected a new subspecies based upon it, Glauconycteris superba sheila. The patterning of this specimen differs in that (1) two white spots are found on each shoulder next to the base of the humerus, (2) the unpigmented areas on the upper surface of the elbow, knee and ankle joints are present, and (3) the ventral interfemoral membrane is a pale gray color. Our newly collected specimen more closely resembles the Ghana specimen, but has only one white spot on each shoulder next to the base of the humerus and lacks an unpigmented area at the base of the ankle (Fig. 2). The recent DRC specimen (Gembu Tungaluna 2012[3]) resembles our South Sudan specimen, but has the unpigmented ankle spots. The only other specimen of Niumbaha superba is from the Ivory Coast (RMCA A9363) and, while cited by Peterson and Smith (1973)[4], it has not been described in the literature and we have not examined it. However, Peterson, in his museum notes, noted that it corresponds to Glauconycteris superba sheila (Peterson, in litt., Royal Ontario Museum notes). Thus, of the five specimens, four appear to have characteristics attributed to the subspecies sheila and only one to the nominate subspecies. However, given the variation seen within the specimens of the subspecies sheila and giventhat the single specimen attributed to the nominate subspecies was captured in relatively close proximity to two specimens that match more closely the pelage patterning described for sheila,we do not recognize sheila as a valid subspecies (see also Simmons 2005[5]). Within species of Glauconycteris, the tendency to produce patterns of spots, stripes and reticulations is pronounced and variable (Rosevear 1965[1]). In Glauconycteris poensis, for example, Hayman and Jones (1950)[6] described “remarkable” variation in the pattern of white shoulder spots and flank stripes, suggesting that variation is normal for this and related species. Further study, ideally based upon the collection and (morphological and genetic) study of additional material from additional localities, will be needed to ascertain whether clear patterns of geographic variation exist within Niumbaha superba and whether multiple subspecies can be recognized.
Notably, our specimen of Niumbaha superba (and that reported by Gembu Tungaluna 2012[3]) was not originally black and white when collected, but rather black and cream/buffy yellow. Hayman (1939[2], 1947) described superba from museum specimens, in which we suspect the color had faded (Rosevear [1965] also noted the “pure white hairs” and included a drawing of Glauconycteris superba sheila, taken from a black and white photograph [from which the original color is thus not clear] of the bat on a tree trunk). Indeed, our specimen, fixed in formalin and stored in ethanol, is now black and white, such that the yellow coloration of the paler fur ornamentation has leached from the fur, and only the images of the freshly collected bat indicate its true color.
Finally, Niumbaha superba is larger than all species of Glauconycteris, as noted by Hayman (1939[2], 1947). Rosevear (1965)[1] subsequently noted the larger body size as well, but also noted that body size measurements are not “very much larger” than Glauconycteris variegata and Glauconycteris argentata, but that the skull is far bigger, with a total skull length greater than 16mm (Table 2; see also discussion below).
Wing morphology: Rosevear (1965)[1] distinguished Glauconycteris from other African Vespertilioninae by its distinctive wing morphometry – noting that phalanx 2 (Ph2) on digit 3 (DIII) is longer than Ph1. Within Glauconycteris, Glauconycteris variegata is perhaps the best studied species and Findley et al. (1972)[7] described it being among the bat species with the highest aspect ratio (wing length/wing width) and the longest wing tips. Wing size and shape represent a compromise between different (and often conflicting) selective forces and the kinematics of bat flight are complex (Norberg and Rayner 1987[8]). Nevertheless, we can say that the long pointed wingtips and high aspect ratio of Glauconycteris variegata suggest relatively maneuverable, low flight speed that might favor feeding in open areas around, but not within clutter (Norberg and Rayner 1987[8]; and see Obrist et al. 1989[9], whose examination of echolocation calls also supported this flight/feeding mode). Niumbaha superba, while retaining Ph2>Ph1 for DIII, diverges from Glauconycteris in that the ratio of Ph2/Ph1 is significantly less extreme (1.15 ± 0.05 SD vs. 1.51 ± 0.12 SD; t = -6.12, df = 31, p < 0.0001; Fig. 4), which has not previously been noted for this taxon. This suggests that Niumbaha is perhaps closer to ‘typical’ vespertilionids in ecomorphological space (for comparison, measurements for Scotophilus are also included in Fig. 4). This difference in wing shape may reflect differences in habitat type and feeding mode (see also the discussion of differences in dentition between Niumbaha and Glauconycteris, below).
Facial features (including the ear): Glauconycteris is distinctive among African vespertilionids in possessing an extremely shortened but broad muzzle in which the nostrils open more or less to the side from a transverse, thick subcylindrical naked pad. On the underlip is found a thickened pair of pads and the lower lip near the corner of the mouth has a fleshy lappet or fold that can be made to extend horizontally (Rosevear 1965[1]). The rostrum is proportionally longer in Niumbaha superba as compared to Glauconycteris, but we have found no mention in the literature of differences in other facial features.We note here that the fleshy lappet is present on the lower lip but that the muzzle appears to be more robust and contains nostrils that open more to the front than to the side (Fig. 3), a more ‘typical’ vespertilionid configuration.
The ears of Glauconycteris sensu stricto are of small to moderate size and rounded with a strong semicircular inner margin that ends basally in a “curiously backwardly projecting lobe” and a pronounced antitragus (Rosevear 1965[1]:273). The tragus is “sickle” or half-moon shaped with a large and broadly triangular basal lobe. In his original description of Niumbaha superba, Hayman (1939)[2] noted that the ears are less rounded and more subquadrangular than in other Glauconycteris (Fig. 3). Rosevear (1965:284-285), noting that his observations were from a dried skin, described the inner margin of the ear of Niumbaha superba sheila as “terminating in a long almost parallel-sided free lobe”, the antitragus as large and semicircular, and the tragus as broader than in other Glauconycteris with a “boldly curved” outer margin and a small acute lobule. Based upon examination of the fresh and subsequent fluid specimen from South Sudan, we generally concur. The “free lobe” at the inner margin of the ear is larger in Niumbaha than in Glauconycteris, but we note that the antitragus is more squared off than semicircular. Additionally, the horizontal cartilaginous ridges in the outer ear margin are pronounced in Niumbaha (especially in the fresh specimen; Fig. 3) relative to Glauconycteris.
Cranial features: Despite placing this bat in Glauconycteris, Hayman (1939:222) noted that the skull was longer and less broad with marked flattening of the rostrum “so that the profile shows an angle at the junction of the brain-case and the rostrum” and (1947:549) and so that there is “considerable lengthening of the infraorbital foramen”; he also noted the presence of proportionally deeper basisphenoid pits (Fig. 5). Rosevear (1965)[1] noted that the skull is significantly larger and more powerful than Glauconycteris sensu stricto and that the upper surface of the rostrum does not rise in an even plane from the incisors to the occiput (as occurs in most Glauconycteris, see skull images of Glauconycteris variegata and Glauconycteris poensis in Fig. 5) but rather is flat or roughly parallel to the upper toothrow. This results in an excavation or “hollowing-out” of the frontal region of the skull (Fig. 5). Lastly, while Glauconycteris have a domed braincase with virtually no sagittal crest, a low crest is present in Niumbaha, where it joins posteriorly with a lambdoidal crest to form a low supraoccipital pyramid (Rosevear 1965[1]).
Niumbaha shares its dental formula and many dental characteristics with Glauconycteris. The dental formula is 2.1.1.3/3.1.2.3 = 32, but Hayman (1939)[2] noted a greater proportional difference in size between the lower i1 and i3 than in Glauconycteris sensu stricto (Fig. 5). As with Glauconycteris, the upper incisor is long and pointed and the upper premolar is long, similar in height to the molars. While Hayman (1947)[10] noted a considerably reduced m3 compared to other (we presume Glauconycteris) species, we do not find this to be the case in our South Sudan specimen. The canines, and especially the upper canine, are considerably more robust (unreduced) in Niumbaha than in Glauconycteris. The size difference between Niumbaha and Glauconycteris presumably allows Niumbaha to take larger, more hard-bodied prey than Glauconycteris, an apparent lepidopteran (moth) specialist (Fenton et al. 1977[11]).
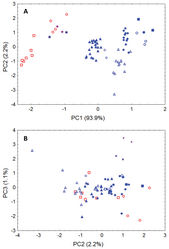
Our principal components analysis of cranial and dental data (based upon measurements listed in Table 2 from Niumbaha, Glauconycteris, and Scotophilus) clearly indicates that the skulls of Niumbaha separate from skulls of species of Glauconycteris, suggesting greater overall ecomorphological resemblance of Niumbaha with medium-sized, less specialized African vespertilionids such as Scotophilus (Fig. 6). The first principal component reflects distinctions in overall skull size and indeed each of the cranial measurements in this analysis is significantly larger for Niumbaha than for Glauconycteris (see Table 2). Beyond size, separation of skulls of Niumbaha from those of Glauconycteris and Scotophilus in combination along the second and third components indicates the morphological isolation of Niumbaha and illustrates consistent differences in skull shape, reflecting (in separation along the third component) the proportionally narrower interorbital dimensions, less dramatic postorbital constriction, longer toothrows, narrowed skull, but widened anterior rostrum in Niumbaha relative to Glauconycteris.
| Variable | PC1 | PC2 | PC3 |
|---|---|---|---|
| Zygomatic breadth | -0.988 | 0.003 | -0.044 |
| Mastoid width | -0.962 | -0.083 | -0.098 |
| Breadth of braincase | -0.940 | -0.218 | 0.082 |
| Height of braincase | -0.969 | -0.137 | -0.020 |
| Interorbital width | -0.970 | -0.109 | -0.160 |
| Postorbital process width | -0.971 | -0.133 | -0.146 |
| Postorbital constriction | -0.489 | -0.726 | 0.449 |
| Width at M3 | -0.977 | 0.035 | 0.064 |
| Maxillary toothrow length (C-M3) | -0.985 | 0.129 | 0.073 |
| Width at upper canines | -0.966 | 0.054 | 0.091 |
| Greatest length of mandible | -0.989 | 0.077 | 0.012 |
| Mandibular toothrow length | -0.983 | 0.130 | 0.054 |
| Eigenvalues | 0.222 | 0.005 | 0.003 |
| Percent variance (%) | 93.9 | 2.1 | 1.1 |
Distribution and habitat
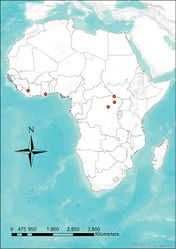
Niumbaha superba has been rarely captured (only five times) but is apparently widely distributed (Fig. 7), being recorded from Ghana, Ivory Coast, Democratic Republic of the Congo and South Sudan. This broad distribution suggests that it is more common than its collection records indicate. Although most species in its apparent sister genus, Glauconycteris,are not well known, at least one species (Glauconycteris variegata) is believed to be a high flier (Obrist et al. 1989[9]), which could translate to poor capture success for Niumbaha, especially if it typically flies at even greater heights. Glauconycteris are found in a variety of habitats, mostly from moist forest zones (Rosevear 1965[1]). We can only speculate that Niumbaha is found in similar habitat types. Neither the description of the first specimen collected in the Democratic Republic of Congo (Hayman 1939[2]) nor that of the second specimen from Ghana, which was “found alive on the ground” (Hayman 1947[10]:550) contain habitat descriptions. However, Rosevear (1965)[1] noted that both locations were in closed forest (though the Ghana location was on the edge of closed forest and a Guinea woodland zone) and Hayman and Hill (1971)[12] noted that both locations are from heavy rain forest. A recent specimen from Democratic Republic of the Congo was mist-net captured in secondary forest (Gembu Tungaluna 2012[3]) and our specimen from South Sudan was mist-net captured on a grassland plateau just above a secondary thicket forest.
Original Description
- Reeder, D; Helgen, K; Vodzak, M; Lunde, D; Ejotre, I; 2013: A new genus for a rare African vespertilionid bat: insights from South Sudan ZooKeys, 285: 89-115. doi
Other References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Rosevear D (1965) The bats of West Africa. Trustees of the British Museum (Natural History), London, United Kingdom.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Hayman R (1939) Two new mammals from the Belgian Congo. Annals and Magazine of Natural History, Series 11, 3: 219–224. doi: 10.1080/03745481.1939.9723594
- ↑ 3.0 3.1 3.2 3.3 Gembu T (2012) Observation 24. Fourth observation of Glauconycteris superba from the Democratic Republic of the Congo. African Bat Conservation News 28: 2-3.
- ↑ Peterson R, Smith D (1973) A new species of Glauconycteris (Vespertilionidae, Chiroptera). Royal Ontario Museum, Life Sciences Occasional Papers 22: 1-9.
- ↑ Simmons N (2005) Order Chiroptera. In: Wilson D Reeder D (Eds). Mammal species of the world. A taxonomic and geographic reference. Johns Hopkins University Press, Baltimore, Maryland, USA: 312-529.
- ↑ Hayman R, Jones T (1950) A note on pattern variation in the vespertilionid Glauconycteris poensis (Gray). Annals and Magazine of Natural History, Series 12, 3: 761–763. doi: 10.1080/00222935008654104
- ↑ Findley J, Studier E, Wilson D (1972) Morphologic properties of bat wings. Journal of Mammalogy 53: 429-444. doi: 10.2307/1379035
- ↑ 8.0 8.1 Norberg U, Rayner J (1987) Ecological morphology and flight in bats (Mammalia, Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society of London 316B: 335–427. doi: 10.1098/rstb.1987.0030
- ↑ 9.0 9.1 Obrist M, Aldridge H, Fenton M (1989) Journal of Mammalogy 70: 828-833. doi: 10.2307/1381721
- ↑ 10.0 10.1 Hayman R (1947) A new race of Glauconycteris superba from West Africa. Annals and Magazine of Natural History, Series 11, 13: 547–550. doi: 10.1080/00222934608654576
- ↑ Fenton M, Boyle N, Harrison T, Oxley D (1977) Activity patterns, habitat use, and prey selection by some African insectivorous bats. Biotropica 9: 73-85. doi: 10.2307/2387662
- ↑ Hayman R, Hill J (1971) Order Chiroptera. In: The mammals of Africa- an identification manual (Meester J, Setzer HW, eds). Smithsonian Institution Press, Washington DC, USA, 1–73.
Images
|