Pestalotiopsis sydowiana
A minireview by G. Hagedorn (with collaborators, see history tab at top)
Contents
Pestalotiopsis sydowiana (Bresadola) Sutton
- Mycological Papers 80:14, 1961
Basionym: Pestalotia sydowiana Bresadola, Hedwigia 35: 32 (1896)
Synonyms (after Guba 1961, Sutton 1961, Nag Raj 1993):
- = Pestalotia rhododendri (D. Sacc.) Guba, Phytopathology 19: 215 (1929) (non Pestalotia rhododendri Westendorp 1858, nom. nud.)
- = Pestalotia epigaeae P. Henning, Notizbl. K. Bot. Gard. Mus. Berlin 3: 40 (1900)
- = Pestalotia macrotricha Klebahn, Myc. Centralbl. 4: 6 (1914)
- = Pestalotia cavendishiae Chardon & Toro, Journal Dept. Agr. Porto Rico 14: 279 (1930)
- = Pestalotia guepini Desm. var. rhododendri Cooke, J. E. Vize, Microf. Brit. 509, 512 (1888, nom. nud.)
- = Pestalotiopsis sydowiana (Bresadola) Zhu, Ge & Xu (1991, a redundant combination)
Potentially erroneous names:
- = The name Pestalotiopsis rhododendri (D. Sacc.) Guba, used by Murugan & al. 2007, is probably a misapplication of Pestalotia rhododendri (D. Sacc.) Guba.
- = Pestalotiopsis rhododendri (Guba) Y. X. Chen, 1994, J. Guangxi Agric. Univ. 13: 125 (cited with full authors and reference, in Wei & al. 2005). The basionym author "Guba" seems to be erroneous. Without authors, in Liu & al. (2007).
- The name Pestalotiopsis rhododendri is not included in indexfungorum. The "J. Guangxi Agric. Univ. 13: 125" is currently not available to us, so the correct citation could not be checked. P. rhododendri without authority is further cited in Wei & al. (2007: Table 2 and 3), Liu & al. (2007: Table 1, citing Genbank AF409986, Fig 2., and p. 33), Jeewon & al. (2003: Table 1, they submitted GenBank AF409986 from Antidesma ghaesembilla, Australia).
- In addition to AF409986 cited above, Genbank also has AY687304, submitted by Wei, Xu and Guo (2004), under Pestalotiopsis rhododendri. No authority information for the name is present in GenBank.
Recommendation: The name Pestalotiopsis rhododendri should presently be avoided. A conspecificity of Pestalotiopsis sydowiana (Bresadola) Sutton with Pestalotia rhododendri - and thus with any Pestalotiopsis rhododendri is very likely.
Type
According to Sutton (1961), the type material of Pestalotia sydowiana from B and BM is very poor; Sutton's description is primarily based on the type of Pestalotia rhododendri.
Morphology
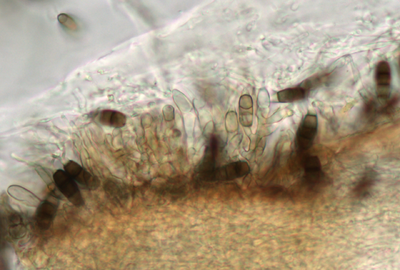
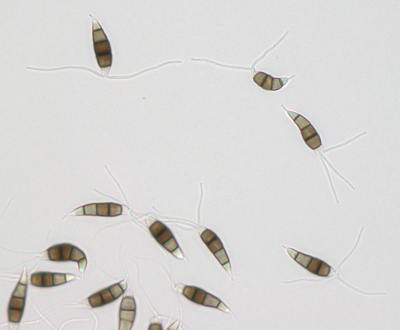
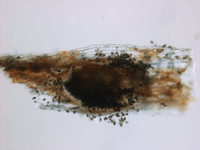
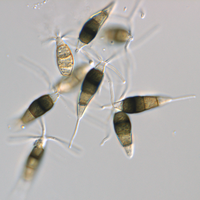
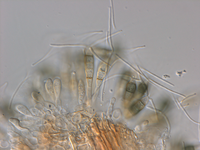
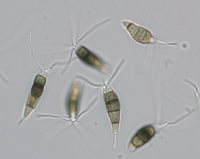
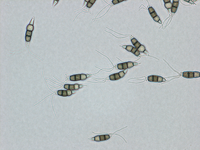
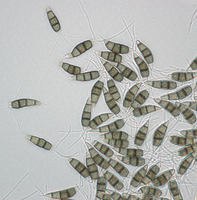
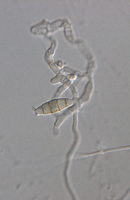
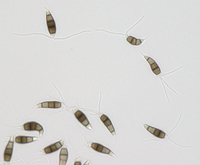
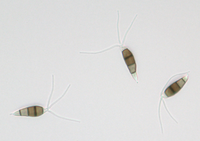
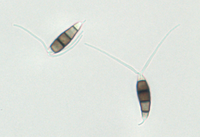
Description modified after Guba (1961) and Sutton (1961): Acervuli abundant, epiphyllous on dried, dead leaves or sometimes associated with light discoloured areas, sparse or densely gregarious with frequently confluent, black spore masses which are more scattered and discrete towards the edge of the colony; globose-lenticular and irregularly erumpent, surrounded by torn epidermis, 100-400 µm diam., seated in ash-gray or brownish spots with reddish margins. Conidiophores arise from the upper cells of the stroma, erect, hyaline, obpyriform, 1-3 µm diameter, about 10 µm long, conidiogenesis at the apex, aseptate with normally 1 (-3) proliferation (after liberation of the conidium, the conidiophore proliferates through the apex to form another conidium). Conidia clavate to fusiform, straight, rarely curved, equilateral, 5-celled, smooth-walled, 23.0-29.0 x 8.0-9.5 (-11) µm, mean 25.0 x 9.0 µm. Apical and basal cells hyaline; apical hyaline cells long and broad cylindric; the basal hyaline cells broad-conic. Median three cells colored, guttulate, together 16-20 µm (mean 18 µm) long, slightly or hardly constricted at the septa, the lowest colored cell is light brown, the upper two cells darker brown (fuliginous or umber). The septum separating the two superior cells is very dark brown to almost black. Median cells in total 17-20 µm (mean 18 µm) long. Apical appendages (2-) 3 (-4), divergent or recurved, hyaline, cylindrical with obtuse apices, 18-40 µm long (mean 22 µm). Basal appendage hyaline, straight or slightly curved, 3-6 µm long (mean 4 µm).
The species was not studied by Nag Raj (1993), who refers to Sutton and Guba.
Illustrations
- Guba 1961: 199, Fig. 67.
- Sutton 1961.
- Murugan & al. 2007
- Remlein-Starosta 2004 - Disease symptoms and condial morphology.
Biology
The fungus, although regularly occurring as a pathogen of hardy ornamentals, mostly Ericaceae, seems to have a wide host spectrum. On artifical inoculation, it acts as a fruit-rot agent of apples (Wollenweber & Hochapfel, Z. Pflanzenkrankheiten 46: 401).
Pestalotiopsis species, including P. sydowiana are known as endophytic fungus (see, e. g., the publications by Wei, Liu and Jeewon). In general, the host specificity within the genus seems to have been overestimated. It is now established that several molecularly distinct species may occur on one host, whereas the same species - as defined on molecular basis - may infect multiple hosts (Jeewon & al. 2004).
Specifically for Pestalotiopsis sydowiana, Hopkins and McQuilken (2000) studied the pathogenicity of 18 isolates of this species and demonstrated that the isolates were not host-specific and infected species of hardy ornamentals other than those from which they were originally isolated.
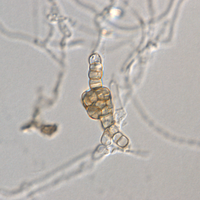
Sutton (1961) confirms for P. sydowiana that only the lowermost of the three median cells (which is the lightest colored) is capable of germination. In a follow-up of Sutton (1961), Muruguan & al. (2007) studied the conidiomatal development of Pestalotiopsis rhododendri.
Similar species
Pestalotiopsis malicola is a pathogen of Rhododendron and other ornamentals known from Japan; the three median colored cells are together only 13-16 µm long.
Symptoms on cultivated Erica darleyensis
| Symptoms on cultivated Erica darleyensis plants | |||||||||
| |||||||||
Material studied
Strain BBA/JKI 72137
Isolated from Arctostaphylos uva-ursi (variety used for pharmaceutical purposes to obtain Arbutin; not ornamental), May 2002, received from Dr. U. Gärber, JKI.
Description on SNA: Conidia 5-celled, 22-27 x 7.0-10.0 µm (mean 24.7 x 8.5 µm, n = 15, measured without appendages); apical and basal cell hyaline; median 3 cells brownish, separating septum between these thick and dark brown (but not distoseptate), 14.5-18 µm (mean 16.0 µm, n = 15); the inferior cell lighter brown, the other two cells darker brown, most often the medium cell is darkest, sometimes both cells equally, and rarely the top cell darker than the medium cell. Apical appendages 3 (rarely 2), hyaline, unbranched. Basal appendage hyaline, single, unbranched, endogenous.
Strain BBA/JKI 72137 on PDA, under continuous near-UV light, 20 °C, after 27 days:
Conidia: (21.7) 25.7–27.8 (29.3) × (6.2) 6.66–7.34 (7.8) µm [n = 30, mean / s. d. = 25.7 / 2.1 × 7 / 0.34]. Length of median three cells: (15.1) 16.5–17.45 (18.3) µm [n = 30, mean / s. d. = 16.5 / 0.95 ]. Basal appendages: (2) 3.5–4.2 (4.9) µm [n = 27, mean / s. d. = 3.5 / 0.7 ]. Apical appendages: (13) 26.3–30.54 (43.7) µm [n = 84, mean / s. d. = 26.3 / 4.24 ]. Two outliers exist in the previous measurements. Removing them, the apical appendage changes to: (13) 25.9–29.27 (33.5) µm [n = 82, mean / s. d. = 25.9 / 3.37 ]
Daily radial growth rate in culture (average of three Petri-dish cultures, measuring and averaging two crossing diameters in each dish, daily rate based on diameter after 7 days minus diameter after 3 days, the calculated diameter includes 5 mm inoculum), on:
- PDA, 20°C, darkness: daily radial growth 4.8 mm; calculated diameter after 7 days: 71.5 mm.
- PDA, 25°C, darkness: daily radial growth 5.0 mm; calculated diameter after 7 days: 75.4 mm.
- PDA, 20°C, continuous near-UV: daily radial growth 3.4 mm; calculated diameter after 7 days: 52 mm.
| Extended depth-of-field images for fructifications in culture | |||||||||
| |||||||||
Strain BBA/JKI 72157
On Erica spec..
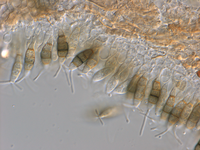
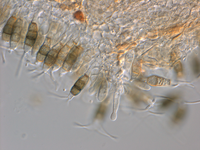
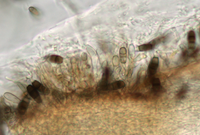
a) on the host (after incubation in moist chamber)
Acervuli on Erica spec. dark brown to black, ca. 150-350 µm in diameter. Conidia 5-celled, median 3 cells always darker, median cells versicolorous: usually top 2 cells darker than lower cell, of the top two sometimes topmost and sometimes median cell darkest. All cells smooth-walled. Conidial length (21-) 26.1 (-30.9) (n=12, min/mean/max.); total length of colored three median cells: (12.5-) 15.3 (-18.1) (n=32, min/mean/max.). Apical appendages 2, 3, or rarely 4; 14-36 µm long; tips not knobbed or spatulate. Basal appendage single unbranched, endogenous, or absent.
| Observations of strain BBA 72157 from naturally infected material | ||||||||||||||||||||||
| ||||||||||||||||||||||
b) on PDA, under continuous near-UV light, 20 °C, after 27 days:
Conidia 5-celled, median 3 cells always darker, median cells versicolorous: usually top 2 cells darker than lower cell, of the top two sometimes topmost and sometimes median cell darkest. All cells smooth-walled, (22.8) 27.5–30.3 (33.4) × (6.2) 6.52–7.48 (7.9) µm [n = 30, mean / s. d. = 27.5 / 2.8 × 7 / 0.48]. Length of median three cells: (13.4) 17.4–19.23 (21.5) µm [n = 30, mean / s. d. = 17.4 / 1.83 ]. Basal appendages single unbranched, endogenous, or absent, (2.9) 4.5–5.19 (5.7) µm [n = 25, mean / s. d. = 4.5 / 0.69 ]. Apical appendages: 2, 3, or rarely 4, (19.4) 28.6–32.29 (37.4) µm [n = 75, mean / s. d. = 28.6 / 3.69 ] long, tips not knobbed or spatulate.
Daily radial growth rate in culture (average of three Petri-dish cultures, measuring and averaging two crossing diameters in each dish, daily rate based on diameter after 7 days minus diameter after 3 days, the calculated diameter includes 5 mm inoculum), on:
- PDA, 20°C, darkness: daily radial growth 5.4 mm; calculated diameter after 7 days: 81.1 mm.
- PDA, 25°C, darkness: daily radial growth ca. 6.3 mm; calculated diameter after 7 days: 88.2 mm.
- PDA, 20°C, continuous near-UV: daily radial growth 5.1 mm; calculated diameter after 7 days: 77 mm.
| Observations from strain BBA 72157 from PDA | ||||||||||
| ||||||||||
| Unclear observations from BBA 72157 | |||||||||
| |||||||||
Strain BBA/JKI 72158
On Calluna vulgaris, Elsass (France); received as strain "Gshm F 103" on PDA from the Forschungsanstalt Geisenheim.
BBA/JKI 72158 on PDA, under continuous near-UV light, 20 °C, after 27 days:
Conidia: (21.7) 25.6–28.31 (31.4) × (6.1) 6.67–7.53 (8) µm [n = 30, mean / s. d. = 25.6 / 2.71 × 7.1 / 0.43]. Length of three median cells: (14) 16.3–17.94 (19.8) µm [n = 30, mean / s. d. = 16.3 / 1.64 ]. Basal appendages: (1.4) 3.5–4.39 (4.9) µm [n = 27, mean / s. d. = 3.5 / 0.89 ]. Apical appendages: (17.2) 27.8–31.51 (36.6) µm [n = 82, mean / s. d. = 27.8 / 3.71 ].
Daily radial growth rate in culture (average of three Petri-dish cultures, measuring and averaging two crossing diameters in each dish, daily rate based on diameter after 7 days minus diameter after 3 days, the calculated diameter includes 5 mm inoculum), on:
- PDA, 20°C, darkness: daily radial growth 5.3 mm; calculated diameter after 7 days: 79.1 mm.
- PDA, 25°C, darkness: daily radial growth 3.9 mm; calculated diameter after 7 days: 59.3 mm.
- PDA, 20°C, continuous near-UV: daily radial growth 4.4 mm; calculated diameter after 7 days: 67.1 mm.
| Observations from strain BBA 72158 from PDA | ||||||||||||||||||
| ||||||||||||||||||
Molecular results
The three strains available at the JKI were sequenced by Hagedorn (unpublished). Based on the ITS 1 and 2 sequences of BBA 72137, 72157 and 72158 many identical or near-identical sequences may be found in Genbank. This indicates either an unclear species concept in need of revision, or the presence of very closely related species for which ITS provides insufficient distinction. Excluding unidentified and clearly erroneous sequences like "EU732733.1", still a variety of candidates remain. These are discussed below:
- Pestalotiopsis sydowiana and Pestalotiopsis rhododendri
- GenBank: AF409970.1 Pestalotiopsis sydowiana from Protea mellifera, S. Africa (Jeewon & al. 2003).
- GenBank: AY687304.1 Pestalotiopsis rhododendri (Guba) Y. X. Chen 1994 "endophyte on leaves of Podocarpus macrophyllus (Thunb.) D. Don. obtained in Zhejiang Province". In Genbank this is noted as Wei, Xu & Guo unpublished: "Molecular phylogenetics of the Pestalotiopsis based on cladistic analyses of ITS1-5.8S-ITS2 sequences". No indepent publication containing the GenBank number could be found. However, the specimen voucher number noted in Genbank (Institute of Biotechnology Herbarium, Zhejiang University PSHI2002Endo381) is cited in: Wei & al. (2005) on p. 488.
- Pestalotiopsis paeoniicola
- GenBank: AY687310.1 Pestalotiopsis paeoniicola, "endophyte on twigs of Podocarpus nagi (Thunb.) Zoll. Et Mor obtained in Zhejiang Province". In Genbank only noted as Wei, Xu & Guo unpublished: "Molecular phylogenetics of the Pestalotiopsis based on cladistic analyses of ITS1-5.8S-ITS2 sequences", this sequence is analyzed in Liu et al. (2007).
- GenBank: EU400221.1 Pestalotiopsis paeoniicola from Pouteria sapota. In GenBank this is noted as direct submission by Gomez-Jaimes & al., titled "First report of fruit rot (Pestalotiopsis paeoniicola) on zapote mamey (Pouteria sapota) in Southwestern Mexico". A published article under this title could not be found. However, the Genbank accession is also cited in Gómez-Jaimes et al. 2009. According to this publication, the identification as Pestalotiopsis paeoniicola occurred on the basis of sequence similarity alone. This sequence therefore provides no further proof of identity of the sequence with the name Pestalotiopsis paeoniicola.
- Pestalotiopsis clavispora
- GenBank: AY682929.1 Pestalotiopsis clavispora, endophyte on twigs of Camellia sinensis O. Ktze obtained in Zhejiang Province. In Genbank only noted as Wei, Xu & Guo unpublished: "Molecular phylogenetics of the Pestalotiopsis based on cladistic analyses of ITS1-5.8S-ITS2 sequences", this sequence is analyzed in Liu et al. (2007).
- GenBank: EU342213.1 Pestalotiopsis clavispora from Vaccinium corymbosum X Vaccinium darrowi (direct GenBank submission by Espinoza & al., titled "Canker and twig dieback of blueberry (Vaccinium spp.) caused by Pestalotiopsis spp. in Chile").
- GenBank: FJ459947.1 Pestalotiopsis clavispora, no host given, China. Direct Genbank submission by Zhang & al. without further information.
- GenBank: FJ459948.1 Pestalotiopsis clavispora, no host given, China. Direct Genbank submission by Zhang & al. without further information.
- GenBank: FJ459946.1 Pestalotiopsis clavispora, no host given, China. Direct Genbank submission by Zhang & al. without further information.
- Other species
- GenBank: AF409971.1, Pestalotiopsis longisetula from Leucospermum sp., Cecily (Leaf spot). The species is also known as causing rot lesions on strawberry fruits. See table 1 in Jeewon & al. (2004).
- GenBank: AF409969.1, Pestalotiopsis leucothoes from Telopea spec., Hawai. (Jeewon & al. 2003).
At least the identifications by the groups of Wei & al. and Jeewon & al. can be considered as applying the best available morphological data. (Wei & al. (2007) state that they identify Pestalotiopsis species "based on their morphological characters (Steyaert, 1949; Guba, 1961; Nag Raj, 1993)"; Jewoon & al. (2004) state: "Taxa used were identified based on the keys provided by Steyaert (1949), Guba (1961) and Nag Rag (1993)".
Temporary Note: In DNA analysis, the following additional species need to be included:
- Pestalotiopsis rhododendri AF409986 (see Liu & al. 2007)
- Pestalotiopsis aquatica AF409956 from Leucospermum sp. (see Jeewon & al. 2004)
- Pestalotiopsis gracilis AF409962 from Scaevola hainanensis (see Jeewon & al. 2004)
Annotated List of References
Morphology and Host range
- Allescher, A. 1903. Kryptogamenflora von Deutschland, Österreich und der Schweiz, Band 7: p. 691
- Full text: "4573. Pestalotiopsis sydowiana Bresad. in Hedw. 1896, p. (32). Sacc. et Sydow, Syll. XIV. p. 1027. -- Sporenlager auf der Blattoberseite, punktförmig, schwarz, hervorbrechend, dicht zerstreut, in einem aschgrauen, eckig-buchtigen, roth begrenzten Flecken nistend; Sporen spindelig, 24-26 µ lang, 3 µ dick, mit vier Querwänden, bei denselben kaum eingeschnürt; die mittleren Zellen dunkel-olivenfarbig, die Endzellen hyalin; am Scheitel mit drei geraden oder bogig zurückgekrümmten 24-38 µ langen Borsten; Stiel kurz, hyalin, 5-7 µ lang, 1 µ dick. An lebenden Blättern von Gaultheria procumbens im botanischen Garten zu Berlin (Sydow). Der Pilz scheint der Pestalotia longiseta am nächsten zu stehen."
- Brandenburger, W. 1985. Parasitische Pilze an Gefäßpflanzen in Europa, Gustav Fischer Verlag, p. 469.
- "Flecken aschgrau oder bräunlich, mit rötlichem Rand; Acervuli, oberseits 100-400 µm Durchmesser; Konidien keulenförmig, 5zellig, 23-29 x 8-11 µm, Scheitelzelle hyalin, mit (2-)3(-4), 18-30 µm langen Anhängseln, Basalzelle mit einem, 3-6 µm langen Anhängsel."
- Farr, D. F.; Bartolome Esteban, H.; Palm, M. E. 1996. Fungi on Rhododendron. A World Reference 193 p. Boone, NC: Parkway Publishers.
- Guba, E. F. 1961. Monograph of Pestalotia and Monochaetia. Harvard University Press. Cambridge Massachusetts USA.
- "Acervuli globose-lenticular, epiphyllous, sparse or densely gregarious, erumpent, surrounded by torn epidermis, 150-300 µm in diameter, seated in ash-gray or brownish spots with reddish margins. Conidia 5-celled, equilateral, fusiform, tapering to both ends, 23-29 x 8-9.5 µm, intermediate colored cells guttulate, 16-19 µm long, slightly or hardly constricted at the septa, the lowest colored cell olivaceous, the upper two darker or umber; apical hyaline cells long and broad cylindric; the basal hyaline cells broad-conic; setulae 3, rarely 4, divergent or recurved, flexuous, 23-40 µm long, pedicels 6-12 µm long. -- On living leaves of Gaultheria procumbens L., Bot. Gard. Berlin, Germany, 1894-95, P. Sydow in Sydow, Mycotheca Marchica No. 4372 (type of P. sydowiana Bres.)"
- Murugan, M.; Gangadevi, V. & Muthumary, J. 2007. Light and transmission electron microscopic studies on conidiomata developmental morphology in Pestalotiopsis rhododendri. Indian Journal of Science and Technology. Vol.1 No.2 p.1-6 (http://www.indjst.org/archive/issue2/Dec07mm.pdf)
- They observe that the species may produce acervular conidiomata on natural hosts, but pycnidial conidiomata when grown in culture. Although Pestalotiopsis sydowiana is mentioned in the paper, it is not discussed why the two species are considered to be distinct, and the authority given for Pestalotiopsis rhododendri: "(D. Sacc.) Guba," is likely to be erroneous.
- Sutton, Brian C. 1961. Mycological Papers, No. 80, Coelomycetes, Part A: Developmental Studies in Pestalotiopsis, Part B: Five Species of Pestalotiopsis.
- "Acervuli abundant, epiphyllous on dried, dead leaves or sometimes associated with light discoloured areas, densely gregarious with frequently confluent, black spore masses which are more scattered and discrete towards the edge of the colony; globose-lenticular and irregularly erumpent, 100-400 µm diam. Conidiophores arise from the upper cells of the stroma, erect, hyaline, obpyriform, 1-3 µm diameter, about 10 µm long, aseptate with up to 3 but normally 1 proliferation. Conidia are formed singly at the apex of the conidiophore which after liberation of the conidium, proliferates through the apex to form another conidium. Conidia clavate, straight, rarely curved, smooth-walled, 5-celled, 23-29 µm(mean 25 µm) long x 8.0-11.0 µm (mean 9.0 µm) wide. Apical and basal cells are hyaline, whilst the 2 superior median coloured cells are dark brown (fuliginous or umber) and the inferior is light brown. The septum separating the two superior cells is very dark brown to almost black. Median cells 17-20 µm (mean 18 µm) long. Apical appendages 2-4, mostly 3, hyaline, cylindrical with obtuse apices, 18-39 µm (mean 22 µm) in length. Basal appendage hyaline, straight or slightly curved, 3-6 µm (mean 4 µm) in length. On dead leaves of Rhododendron hybridum and R. ponticum, Italy, and living leaves of Gaultheria procumbens, Germany. Isolated from Erica caffra, Great Britain."
Molecular Identification and Phylogenetics
- Gómez-Jaimes, Rafael; Nieto-Ángel, Daniel; Téliz-Ortiz, Daniel; Mora-Aguilera, J. Antonio; Martínez-Damián, M. Teresa & Vargas-Hernández, Mateo 2009. Evaluación de la calidad e incidencia de hongos en frutos refrigerados de zapote mamey (Pouteria sapota (Jacq.) H. E. Moore and Stearn (Evaluation of refrigerated sapote mamey [Pouteria sapota (Jacq.) H. E. Moore and Stearn] quality and incidence of fungi). Agrociencia 43: 37-48.
- Identification as Pestalotiopsis paeoniicola occurred on the basis of sequence similarity alone; however as shown here multiple species names would qualify, all having the same sequence associated with it.
- Jeewon, R., Liew, E. C. Y. Simpson, J. A. Hodgkiss, I. J. & Hyde K. D. 2003. Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Molecular Phylogenetics and Evolution 27: 372-383. (doi)
- Based on strains having been previously identified as P. sydowiana and P. rhododendri - which were found to have separate sequences -, they accept both species, but without detailed discussion or reasoning.
- Jeewon, R., Liew, E. C. Y. & Hyde K. D. 2004. Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal Diversity 17: 39-55. [1]
- A strain of P. sydowiana is included; P. rhododendri not mentioned.
- Lee, Seonju; Crous, Pedro W. & Wingfield, Michael J. 2006. Pestalotioid fungi from Restionaceae in the Cape Floral Kingdom. Studies in Mycology 55: 175–187. http://www.cbs.knaw.nl/publications/55/11%20Pestalotioid%20fungi%20from%20Restionaceae%20in%20the%20Cape%20Floral%20Kingdom.pdf
- Genus concepts of neighboring genera. They cite Pestalotiopsis rhododendri for GenBank AF409968 (in Genbank as "Pestalotiopsis sp. HKUCC 8323").
- Liu, A. R., Xu, T. and Guo, L.-D. 2007. Molecular and morphological description of Pestalotiopsis hainanensis sp. nov., a new endophyte from a tropical region of China. Fungal Diversity 24: 23-36.
- Wei, J. G.; Xu, T. Guo, L.-D. & Pan, X.-Hu 2005. Endophytic Pestalotiopsis species from Southern China. Mycostema 24 (4): 481-493.
- Reference to the specimen number of AY687304.1 Pestalotiopsis rhododendri, basis for its morphological identification, but sequences are not discussed.
- Wei, J. G., Xu, T., Guo, L.-D., Liu, A. R. Zhang, Y. & Pan, X. H. 2007. Endophytic Pestalotiopsis species associated with plants of Podocarpaceae, Theaceae and Taxaceae in southern China. Fungal Diversity 24: 55-74.)
Biology and Control
- Hopkins, K. E. & McQuilken, M. P. 2000. Characteristics of Pestalotiopsis associated with hardy ornamental plants in the UK. European Journal of Plant Pathology 106: 77–85. (http://www.springerlink.com/index/KJ228340Q3P7P104.pdf)
- The authors studied the pathogenicity of 18 isolates of Pestalotiopsis sydowiana and demonstrated that the isolates were not host-specific and able to infect a wide range of hardy ornamentals.
- McQuilken, M. P. & Hopkins, K. E. 2001. Sources, survival and management of Pestalotiopsis sydowiana on Calluna vulgaris nurseries. Crop Protection 20 (7): 591-597.
- McQuilken, M. P., Litterick, A. M. & Hopkins, K. E. 1997. Evaluation of fungicides against Pestalotiopsis sydowiana on Calluna vulgaris and Rhododendron. Tests of Agrochemicals and Cultivars (18): 20-21.
- (not seen)
- McQuilken, M. P. & Hopkins, K. E. 2004. Biology and integrated control of Pestalotiopsis on container-grown ericaceous crops. Pest Management Science 60 (2): 135-142, (doi)
- They confirm the lack of host specificity for P. sydowiana; report growth optimum temperature of three selected isolates as 20–25 °C, little or no growth < 5 or > 30 °C; optimum acidity at pH 5.5 (max. pH 2.6-8.6). Several disease management methods (irrigation, flooring/pot disinfection and fungicide application) were studied for potted plants of Calluna vulgaris. In addition to fungicide-treatment (five-spray program of alternating Prochloraz and Carbendazim) watering by sub-irrigation compared with watering from overhead was beneficial, as were single and combined treatments of flooring/pot disinfection (hydrogen peroxide/peracetic acid).
- Remlein-Starosta, Dorota 2004 Pestalotiopsis associated with Erica spp. ornamental plants in nurseries near Poznań - increasing problem, Journal of Plant Protection Research, Vol. 44, No. 4 (2004) (http://journals.indexcopernicus.com/fulltxt.php?ICID=446487)
- The source of newly occurring, increasingly damaging infection of ericaceous ornamental plants was identified as Pestalotiopsis sydowiana, newly noted in Poland.