Difference between revisions of "Orthopagus"
m (1 revision imported) |
m (Imported from Zoosystematics and Evolution) |
(No difference)
| |
Revision as of 16:46, 6 August 2018
| Notice: | This page is derived from the original publication listed below, whose author(s) should always be credited. Further contributors may edit and improve the content of this page and, consequently, need to be credited as well (see page history). Any assessment of factual correctness requires a careful review of the original article as well as of subsequent contributions.
If you are uncertain whether your planned contribution is correct or not, we suggest that you use the associated discussion page instead of editing the page directly. This page should be cited as follows (rationale):
Citation formats to copy and paste
BibTeX: @article{Song2018ZoosystematicsandEvolution94, RIS/ Endnote: TY - JOUR Wikipedia/ Citizendium: <ref name="Song2018Zoosystematics and Evolution94">{{Citation See also the citation download page at the journal. |
Ordo: Hemiptera
Familia: Dictyopharidae
Name
Orthopagus Uhler, 1897 – Wikispecies link – Pensoft Profile
- Anagnia Stål, 1861: 149. Type species: Flatasplendens Germar, 1830; by original designation and monotypy. Preoccupied by Anagnia Walker, 1854: 446 (Lepidoptera: Erebidae).
- Orthopagus Uhler, 1897: 278; Melichar 1912[1]: 57. Type species: Orthopaguslunulifer Uhler, 1897; by original designation and monotypy.
- Udugama Melichar, 1903: 27; Distant 1906[2]: 249. Type species: Udugamaexoleta Melichar, 1903; by original designation and monotypy. Synonymized with Orthopagus by Oshanin 1908[3]: 444.
- Kareol Kirkaldy, 1904: 279. Replacement name for Anagnia Stål. Synonymized with Udugama by Distant 1906[2]: 249.
Diagnosis
Orthopagus can be distinguished from other genera in the Orthopagini by the following combination of characters: cephalic process short, truncated in front in dorsal view; vertex with lateral carinae strongly ridged and sub-parallel in basal half, slightly constricted at anterior margin of eyes, median carina sharp and complete; frons with intermediate carinae approaching frontoclypeal suture, median carina complete; pronotum with intermediate carinae distinct in basal half; mesonotum with lateral carinae curving anteriad towards median carina; forewings with a wide sublunate streak on distal half of wing, transverse veins sparse, pterostigmal area with 2–4 cells; fore femora flattened and dilated, with a large and blunt spine near apex; hind tibiae with seven apical teeth; phallobase with inflated membranous paired lobes, with or without numerous small superficial spines.
Description
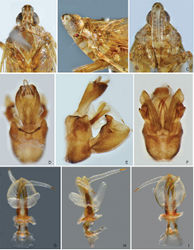
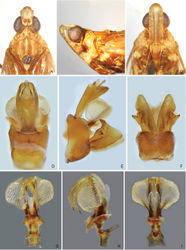
Adult. General colour of body brownish ochraceous to dark brown marbled, with pale green and reddish ochraceous streaks on dorsum (Figs 1A–B, 2A–L). Females distinctly darker than males. Head pale ochraceous with dark brown markings on vertex and frons the extent of which varies among species. Clypeus pale ochraceous basally, with two small dark spots at frontoclypeal suture on each side of median carina, apical half dark brown. Pronotum brownish ochraceous to dark brown, median carina and spots on lateral marginal areas and paranotal lobes pale ochraceous. Mesonotum dark brown, median and lateral carinae, and lateral marginal areas flavescent or greenish. Forewing veins light ochraceous, membrane hyaline to translucent with three dark brown markings: (i) a large sublunate streak extending along the posterio–apical margin from basal portion of areola postica across apical portions of cells of the medial area to the apex of RP vein; (ii) triangular patch on pterostigma, extending also into radial area (C1 cell) and rarely more mesiad as a dark streak along nodal line; (iii) streak along postclaval wing margin filling out whole inner claval cell (Figs 2A–L, 3A–F). Hind wing veins ochraceous, membrane clear, with a more or less developed dark brown marking along the apical portion of CuA1 vein. Legs pale to dark brown: femora dull ochraceous to fuscous, banded and marked with ivory white; fore and mid tibiae yellowish green to ochraceous with two dark brown transverse bands medially; hind tibiae yellowish green (pale ochraceous in old dry-mounted specimens), with base and apex including lateral and apical spines fuscous; fore and mid tarsi fuscous, hind tarsi ochraceous. Abdomen dorsally and ventrally ochraceous to dark brown, with dark brown or pale ochraceous spots and stripes of various sizes and shape. Head (Figs 4A–C, 5A–C, 6A–C, 8A–C, 9A–C, 10A–C) usually produced in a short and stout cephalic process. Vertex (Figs 4A, 5A, 6A, 8A, 9A, 10A) moderately broad, basal width slightly greater than transverse diameter of eyes in dorsal view, posterior plane elevated above pronotum; lateral carinae strongly ridged, foliaceous, and sub-parallel in basal half, slightly constricted at anterior margin of eyes, broadly convex at apex; posterior margin ridged, concave, forming angle of 80–90°; median carina sharp and complete. Frons (Figs 4C, 5C, 6C, 8C, 9C, 10C) with lateral carinae ridged, nearly parallel, slightly expanded outward below antennae; intermediate carinae slightly converging posteriad and approaching frontoclypeal suture; median carina distinct and complete; basal margin of frons projecting anteriad of apex of vertex. Postclypeus and anteclypeus (Figs 4C, 5C, 6C, 8C, 9C, 10C) convex medially, with distinct median carina. Rostrum long, surpassing base of hind femora; basal segment nearly equal to distal one. Compound eyes large and globose. Ocelli relatively large, reddish. Antennae with very small scape; pedicel large and subglobular, with more than 50 distinct sensory plaque organs distributed over entire surface; flagellum long, setuliform. Pronotum (Figs 4A, 5A, 6A, 8A, 9A, 10A) distinctly shorter than mesonotum at midline, anterior margin angularly convex medially, lateral marginal areas straight and sloping down with two long longitudinal carinae on each side, posterior margin concave, forming obtuse angle (100–120°); intermediate carinae distinct in basal half, strongly diverging laterad; median carina sharp and elevated, with a large lateral pit on each side. Mesonotum (Figs 4A, 5A, 6A, 8A, 9A, 10A) tricarinate on disc, lateral carinae converging anteriad towards median carina. Forewings (Fig. 3A–F) hyaline, ratio of length to width about 3:1; venation with sparse transverse veins; MP bifurcating MP1+2 and MP3+4 near middle and beyond CuA; number of apical cells between R and CuA equal to 14; Pcu and A1 veins fused into a long Pcu+A1 vein at apical 1/5 in clavus; pterostigmal area clear, with 2–4 cells. Legs moderately long; fore femora flattened and dilated, with a large and blunt spine near apex; hind tibiae with 5–7 (mostly six) lateral spines and seven apical teeth; hind tarsomeres I with 18–20 and tarsomeres II with 12–14 apical teeth, respectively. Male genitalia. Pygofer (Figs 4D–F, 5D–F, 6D–F, 8D–F, 9D–F, 10D–F) in lateral view distinctly wider ventrally than dorsally, dorsal margin slightly excavated to accommodate segment X, dorso-posterior margins angular, produced into a distinct lobe which is short and broad or larger and tooth-like. Gonostyles (Figs 4E–F, 5E–F, 6E–F, 8E–F, 9G, 10E–F) symmetrical, with narrow base, expanded toward apex, broadest at apical fourth; dorsal margin with a claw-like, apically sclerotised process directed dorsad, outer dorsal edge with a spiny hook-like sclerotised process near middle directed ventrad. Aedeagus (Figs 4G–I, 5G–I, 6G–I, 8G–I, 9H–J, 10G–I) with one pair of elongate endosomal processes extended from phallobase posteriad and strongly curved dorso-anteriad or laterad; these processes are membranous, acute apically and smooth or bearing numerous minute spines over their entire surface; phallobase sclerotised and pigmented basally, membranous and inflated apically, with paired lobes. Segment X (Figs 4D–E, 5D–E, 6D–E, 8D–E, 9D–E, 10D–E) large, in dorsal view with apex deeply excavated to accommodate anal style; anal style elongate and large.
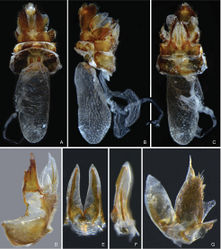
Female genitalia. Gonocoxae VIII (Fig. 7D) with two membranous and flattened endogonocoxal processes (Gxp) on endogonocoxal lobe: Gxp1 large and elongate, with a long sclerotized plate in it; Gxp2 smaller and shorter. Gonapophyses VIII (Fig. 7D) with anterior connective lamina large and sclerotized, with seven teeth of varying sizes and shapes. Gonapophyses IX (Fig. 7E–F) with posterior connective lamina triangular, symmetrical, fused with intergonocoxal plate at base; intergonocoxal plate extended cephalad into genital cavity, forming wall of gonospiculum. Gonoplacs (Fig. 7G) with two lobes homologous; lateral lobe large and moderately sclerotized, with long setae at apex; the posterior lobe membranous, containing long sclerotized plate. Segment X (Fig. 7A) large and broad in dorsal view, apex deeply excavated to accommodate anal style; anal style large and elongate. Female ectodermal genital ducts ditrysian. Bursa copulatrix (Fig. 7A–C) superficially membranous, regularly gridded, without sclerotized ornamentations. A pair of large digitiform glands (Fig. 7B) branched at anterior extremity of the anterior vagina on each side of the spermatheca. Spermatheca (Fig. 7B) divided clearly into five parts: orificium receptaculi, ductus receptaculi, diverticulum ductus, pars intermedialis, and glandula apicalis.
Fifth instar nymph. See Yang and Yeh (1994)[4] for a detailed description.
Diversity and distribution
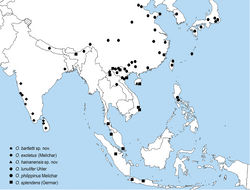
Orthopagus is revised here to include six valid species (see below). The species of the genus are widely distributed in the Oriental and eastern Palaearctic regions from India in the southwest to Japan in the northeast (Fig. 11).
Nomenclatorial remark on Dictyopharaindiana Walker, 1851
The identity of one more available species name belonging to Orthopagus could not be sufficiently cleared during this study: Dictyophora [sic] indiana Walker, 1851: 310 described from India (without more precise locality data). This name was synonymized under Anagniasplendens (Germar) (now Orthopagussplendens) by Stål (1861)[5]: 149. However, this synonymy is considered doubtful here because the original description of D.indiana lacks diagnostic information and illustrations which would enable recognition of its species identity and the single available type specimen of D.indiana (deposited in BMNH) could not be directly examined during this study due to its very poor condition which did not allow its sending out for a loan. Based on a photograph kindly provided by M. D. Webb (BMNH), the type specimen belongs to an Orthopagus species but it lacks the abdomen and its head has been partly damaged. As the details of the male genitalia and the coloration and proportions of the head, i.e. the characters which are missing or damaged in the type, are the most reliable diagnostic morphological characters of Orthopagus species, it is not certain that even a direct examination of the type would help to solve the identity of D.indiana. Therefore, it is proposed here to treat Dictyopharaindiana as a nomen dubium. Currently, four Orthopagus species are known from the Indian subcontinent: O.bartletti sp. n., O.exoletus, O.lunulifer and O.splendens, of which O.exoletus is the most widespread (Fig. 11).
Taxon Treatment
- Song, Z; Malenovský, I; Chen, J; Deckert, J; Liang, A; 2018: Taxonomic review of the planthopper genus Orthopagus (Hemiptera, Fulgoromorpha, Dictyopharidae), with descriptions of two new species Zoosystematics and Evolution, 94(2): 369-391. doi
Images
|
Other References
- ↑ Melichar L (1912) Monographie der Dictyophorinen (Homoptera). Abhandlungen der K. K.Zoologisch-Botanischen Gesellschaft in Wien7(1): 1–221.
- ↑ 2.0 2.1 Distant W (1906) The Fauna of British India, including Ceylon and Burma. Rhynchota Vol. III (Heteroptera-Homoptera).Taylor & Francis, London, 503 pp.
- ↑ Oshanin V (1908) Verzeichnis der palaearktischen Hemipteren mit besonderer Berücksichtung ihrer Verteilung im Russischen Reiche. II. Band. Homoptera. III. Lieferung. Annuaire du Musée Zoologique de l’Académie Impériale des Sciences de St.-Pétersbourg13: 385–492.
- ↑ Yang C, Yeh W (1994) Nymphs of Fulgoroidea (Homoptera: Auchenorrhyncha) with descriptions of two new species and notes on adults of Dictyopharidae.Chinese Journal of Entomology Special Publication8: 1–189.
- ↑ Stål C (1861) Miscellanea hemopterologica. Entomologische Zeitung.Herausgegeben von dem entomologischen Vereine zu Stettin22: 129–153. https://doi.org/10.1111/zoj.12401